Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation
- PMID: 19455146
- PMCID: PMC2714252
- DOI: 10.1038/sj.bjc.6605060
Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation
Abstract
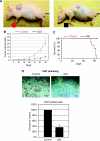
The study shows constitutive activation of the Notch pathway in various types of malignancies. However, it remains unclear how the Notch pathway is involved in the pathogenesis of osteosarcoma. We investigated the expression of the Notch pathway molecules in osteosarcoma biopsy specimens and examined the effect of Notch pathway inhibition. Real-time PCR revealed overexpression of Notch2, Jagged1, HEY1, and HEY2. On the other hand, Notch1 and DLL1 were downregulated in biopsy specimens. Notch pathway inhibition using gamma-secretase inhibitor and CBF1 siRNA slowed the growth of osteosarcomas in vitro. In addition, gamma-secretase inhibitor-treated xenograft models exhibited significantly slower osteosarcoma growth. Cell cycle analysis revealed that gamma-secretase inhibitor promoted G1 arrest. Real-time PCR and western blot revealed that gamma-secretase inhibitor reduced the expression of accelerators of the cell cycle, including cyclin D1, cyclin E1, E2, and SKP2. On the other hand, p21(cip1) protein, a cell cycle suppressor, was upregulated by gamma-secretase inhibitor treatment. These findings suggest that inhibition of Notch pathway suppresses osteosarcoma growth by regulation of cell cycle regulator expression and that the inactivation of the Notch pathway may be a useful approach to the treatment of patients with osteosarcoma.
Figures



References
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 - PubMed
-
- Bettenhausen B, Hrabe de Angelis M, Simon D, Guenet JL, Gossler A (1995) Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila delta. Development 121: 2407–2418 - PubMed
-
- Bin Hafeez B, Adhami VM, Asim M, Siddiqui IA, Bhat KM, Zhong W, Saleem M, Din M, Setaluri V, Mukhtar H (2009) Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin Cancer Res 15: 452–459 - PMC - PubMed
-
- Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE (2005) Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi's sarcoma tumor cells. Oncogene 24: 6333–6344 - PubMed
-
- Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, Roberts JR, Carbone DP (2000) Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst 92: 1355–1357 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

