Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep
- PMID: 19455148
- PMCID: PMC2834335
- DOI: 10.1038/mp.2009.46
Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep
Abstract
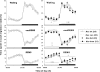
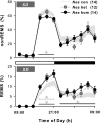
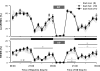
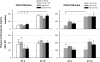
Impaired sleep and enhanced stress hormone secretion are the hallmarks of stress-related disorders, including major depression. The central neuropeptide, corticotropin-releasing hormone (CRH), is a key hormone that regulates humoral and behavioral adaptation to stress. Its prolonged hypersecretion is believed to play a key role in the development and course of depressive symptoms, and is associated with sleep impairment. To investigate the specific effects of central CRH overexpression on sleep, we used conditional mouse mutants that overexpress CRH in the entire central nervous system (CRH-COE-Nes) or only in the forebrain, including limbic structures (CRH-COE-Cam). Compared with wild-type or control mice during baseline, both homozygous CRH-COE-Nes and -Cam mice showed constantly increased rapid eye movement (REM) sleep, whereas slightly suppressed non-REM sleep was detected only in CRH-COE-Nes mice during the light period. In response to 6-h sleep deprivation, elevated levels of REM sleep also became evident in heterozygous CRH-COE-Nes and -Cam mice during recovery, which was reversed by treatment with a CRH receptor type 1 (CRHR1) antagonist in heterozygous and homozygous CRH-COE-Nes mice. The peripheral stress hormone levels were not elevated at baseline, and even after sleep deprivation they were indistinguishable across genotypes. As the stress axis was not altered, sleep changes, in particular enhanced REM sleep, occurring in these models are most likely induced by the forebrain CRH through the activation of CRHR1. CRH hypersecretion in the forebrain seems to drive REM sleep, supporting the notion that enhanced REM sleep may serve as biomarker for clinical conditions associated with enhanced CRH secretion.
Figures
Similar articles
-
Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior.Mol Psychiatry. 2008 Nov;13(11):1028-42. doi: 10.1038/mp.2008.51. Epub 2008 May 13. Mol Psychiatry. 2008. PMID: 18475271
-
REM sleep alteration and depression.Arch Ital Biol. 2014 Jun-Sep;152(2-3):111-7. doi: 10.12871/000298292014236. Arch Ital Biol. 2014. PMID: 25828683 Review.
-
Central deficiency of corticotropin-releasing hormone receptor type 1 (CRH-R1) abolishes effects of CRH on NREM but not on REM sleep in mice.Sleep. 2010 Apr;33(4):427-36. doi: 10.1093/sleep/33.4.427. Sleep. 2010. PMID: 20394311 Free PMC article.
-
Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice.J Neurosci. 2010 Feb 17;30(7):2571-81. doi: 10.1523/JNEUROSCI.4470-09.2010. J Neurosci. 2010. PMID: 20164342 Free PMC article.
-
Sleep and the hypothalamo-pituitary-adrenocortical system.Sleep Med Rev. 2002 Apr;6(2):125-38. doi: 10.1053/smrv.2001.0159. Sleep Med Rev. 2002. PMID: 12531148 Review.
Cited by
-
Relaxin-3/RXFP3 networks: an emerging target for the treatment of depression and other neuropsychiatric diseases?Front Pharmacol. 2014 Mar 21;5:46. doi: 10.3389/fphar.2014.00046. eCollection 2014. Front Pharmacol. 2014. PMID: 24711793 Free PMC article. Review.
-
Identification of preoptic sleep neurons using retrograde labelling and gene profiling.Nature. 2017 May 25;545(7655):477-481. doi: 10.1038/nature22350. Epub 2017 May 17. Nature. 2017. PMID: 28514446 Free PMC article.
-
Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning?Nat Rev Drug Discov. 2012 May 18;11(6):462-78. doi: 10.1038/nrd3702. Nat Rev Drug Discov. 2012. PMID: 22596253 Review.
-
Stress-induced visceral pain: toward animal models of irritable-bowel syndrome and associated comorbidities.Front Psychiatry. 2015 Feb 16;6:15. doi: 10.3389/fpsyt.2015.00015. eCollection 2015. Front Psychiatry. 2015. PMID: 25762939 Free PMC article. Review.
-
Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory.J Biol Chem. 2010 May 28;285(22):16553-61. doi: 10.1074/jbc.M109.090084. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20353942 Free PMC article.
References
-
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. - PubMed
-
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses. Brain Res Rev. 1990;15:71–100. - PubMed
-
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. - PubMed
-
- Friess E, Wiedemann K, Steiger A, Holsboer F. The hypothalamic-pituitary-adrenocortical system and sleep in man. Adv Neuroimmunol. 1995;5:111–125. - PubMed
-
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

