A novel cross-disciplinary multi-institute approach to translational cancer research: lessons learned from Pennsylvania Cancer Alliance Bioinformatics Consortium (PCABC)
- PMID: 19455246
- PMCID: PMC2675833
A novel cross-disciplinary multi-institute approach to translational cancer research: lessons learned from Pennsylvania Cancer Alliance Bioinformatics Consortium (PCABC)
Abstract
Background: The Pennsylvania Cancer Alliance Bioinformatics Consortium (PCABC, http://www.pcabc.upmc.edu) is one of the first major project-based initiatives stemming from the Pennsylvania Cancer Alliance that was funded for four years by the Department of Health of the Commonwealth of Pennsylvania. The objective of this was to initiate a prototype biorepository and bioinformatics infrastructure with a robust data warehouse by developing a statewide data model (1) for bioinformatics and a repository of serum and tissue samples; (2) a data model for biomarker data storage; and (3) a public access website for disseminating research results and bioinformatics tools. The members of the Consortium cooperate closely, exploring the opportunity for sharing clinical, genomic and other bioinformatics data on patient samples in oncology, for the purpose of developing collaborative research programs across cancer research institutions in Pennsylvania. The Consortium's intention was to establish a virtual repository of many clinical specimens residing in various centers across the state, in order to make them available for research. One of our primary goals was to facilitate the identification of cancer-specific biomarkers and encourage collaborative research efforts among the participating centers.
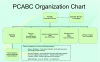
Methods: The PCABC has developed unique partnerships so that every region of the state can effectively contribute and participate. It includes over 80 individuals from 14 organizations, and plans to expand to partners outside the State. This has created a network of researchers, clinicians, bioinformaticians, cancer registrars, program directors, and executives from academic and community health systems, as well as external corporate partners - all working together to accomplish a common mission. The various sub-committees have developed a common IRB protocol template, common data elements for standardizing data collections for three organ sites, intellectual property/tech transfer agreements, and material transfer agreements that have been approved by each of the member institutions. This was the foundational work that has led to the development of a centralized data warehouse that has met each of the institutions' IRB/HIPAA standards.
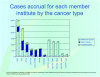
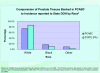
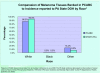
Results: Currently, this "virtual biorepository" has over 58,000 annotated samples from 11,467 cancer patients available for research purposes. The clinical annotation of tissue samples is either done manually over the internet or semi-automated batch modes through mapping of local data elements with PCABC common data elements. The database currently holds information on 7188 cases (associated with 9278 specimens and 46,666 annotated blocks and blood samples) of prostate cancer, 2736 cases (associated with 3796 specimens and 9336 annotated blocks and blood samples) of breast cancer and 1543 cases (including 1334 specimens and 2671 annotated blocks and blood samples) of melanoma. These numbers continue to grow, and plans to integrate new tumor sites are in progress. Furthermore, the group has also developed a central web-based tool that allows investigators to share their translational (genomics/proteomics) experiment data on research evaluating potential biomarkers via a central location on the Consortium's web site.
Conclusions: The technological achievements and the statewide informatics infrastructure that have been established by the Consortium will enable robust and efficient studies of biomarkers and their relevance to the clinical course of cancer. Studies resulting from the creation of the Consortium may allow for better classification of cancer types, more accurate assessment of disease prognosis, a better ability to identify the most appropriate individuals for clinical trial participation, and better surrogate markers of disease progression and/or response to therapy.
Figures




Similar articles
-
A decade of experience in the development and implementation of tissue banking informatics tools for intra and inter-institutional translational research.J Pathol Inform. 2010 Aug 10;1:12. doi: 10.4103/2153-3539.68314. J Pathol Inform. 2010. PMID: 20922029 Free PMC article.
-
The project data sphere initiative: accelerating cancer research by sharing data.Oncologist. 2015 May;20(5):464-e20. doi: 10.1634/theoncologist.2014-0431. Epub 2015 Apr 15. Oncologist. 2015. PMID: 25876994 Free PMC article.
-
An informatics model for tissue banks--lessons learned from the Cooperative Prostate Cancer Tissue Resource.BMC Cancer. 2006 May 5;6:120. doi: 10.1186/1471-2407-6-120. BMC Cancer. 2006. PMID: 16677389 Free PMC article.
-
American Association for Cancer Research Project Genomics Evidence Neoplasia Information Exchange: From Inception to First Data Release and Beyond-Lessons Learned and Member Institutions' Perspectives.JCO Clin Cancer Inform. 2018 Dec;2:1-14. doi: 10.1200/CCI.17.00083. JCO Clin Cancer Inform. 2018. PMID: 30652542 Free PMC article. Review.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
Cited by
-
The development and deployment of Common Data Elements for tissue banks for translational research in cancer - an emerging standard based approach for the Mesothelioma Virtual Tissue Bank.BMC Cancer. 2008 Apr 8;8:91. doi: 10.1186/1471-2407-8-91. BMC Cancer. 2008. PMID: 18397527 Free PMC article.
-
Common data elements of breast cancer for research databases: A systematic review.J Family Med Prim Care. 2020 Mar 26;9(3):1296-1301. doi: 10.4103/jfmpc.jfmpc_931_19. eCollection 2020 Mar. J Family Med Prim Care. 2020. PMID: 32509607 Free PMC article. Review.
-
To Share or Not to Share? A Survey of Biomedical Researchers in the U.S. Southwest, an Ethnically Diverse Region.PLoS One. 2015 Sep 17;10(9):e0138239. doi: 10.1371/journal.pone.0138239. eCollection 2015. PLoS One. 2015. PMID: 26378445 Free PMC article.
-
Big Data: the challenge for small research groups in the era of cancer genomics.Br J Cancer. 2015 Nov 17;113(10):1405-12. doi: 10.1038/bjc.2015.341. Epub 2015 Oct 22. Br J Cancer. 2015. PMID: 26492224 Free PMC article. Review.
-
Clinical research data warehouse governance for distributed research networks in the USA: a systematic review of the literature.J Am Med Inform Assoc. 2014 Jul-Aug;21(4):730-6. doi: 10.1136/amiajnl-2013-002370. Epub 2014 Mar 28. J Am Med Inform Assoc. 2014. PMID: 24682495 Free PMC article.
References
-
- Becich MJ. The role of the pathologist as tissue refiner and data miner: the impact of functional genomics on the modern pathology laboratory and the critical roles of pathology informatics and bioinformatics. Mol. Diagn. 2000;5(4):287–299. - PubMed
-
- Eiseman E.Rand Corporation.: Case studies of existing human tissue repositories: “best practices” for a biospecimen resource for the genomic and proteomic era (http://www.rand.org/publications/MG/MG120/). Santa Monica, CA: RAND; 2003
-
- Gilbertson JR, Gupta R, Nie Y, Patel AA, Becich MJ.2004Automated clinical annotation of tissue bank specimens Medinfo 11(Pt 1)607–610. - PubMed
-
- Master Settlement Agreement [http://www.attorneygeneral.gov/uploadedFiles/Consumers/msa.pdf]
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
