Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes
- PMID: 19455346
- PMCID: PMC3044602
- DOI: 10.1007/s00401-009-0547-7
Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes
Abstract
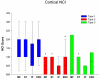
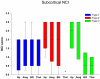
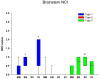
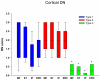
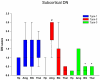
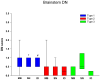
Frontotemporal lobar degeneration (FTLD) can be classified as tau-positive (FTLD-tau) and tau-negative FTLD. The most common form of tau-negative FTLD is associated with neuronal inclusions that are composed of TAR DNA-binding protein 43 (TDP-43) (FTLD-TDP). Recent evidence suggests that FTLD-TDP can be further subdivided into at least three major histologic variants based on patterns of TDP-43 immunoreactive neuronal cytoplasmic inclusions (NCI) and dystrophic neurites (DN) in neocortex and hippocampus. The aim of this study was to extend the histologic analysis to other brain regions and to determine if there were distinct clinical and pathologic characteristics of the FTLD-TDP subtypes. Thirty-nine FTLD-TDP cases were analyzed (Mackenzie type 1 n = 24, Mackenzie type 2 n = 9, Mackenzie type 3 n = 6). There was a highly significant association between clinical syndrome and FTLD-TDP subtype, with progressive non-fluent aphasia associated with type 1, semantic dementia with type 2, and behavioral variant frontotemporal dementia with types 1, 2 and 3. Semi-quantitative analysis of NCI and DN demonstrated different patterns of involvement in cortical, subcortical and brainstem areas that were characteristic for each of the three types of FTLD-TDP. Type 1 had a mixture of NCI and DN, as well as intranuclear inclusions in most cases and TDP-43 pathology at all levels of the neuraxis, but less in brainstem than supratentorial structures. Type 2 cases were characterized by predominance of long, thick DN in the cortex, as well as numerous NCI in hippocampus, amygdala and basal ganglia, but virtually no NCI and only sparse DN in diencephalon and brainstem. Type 3 had a paucity of DN at all levels of the neuraxis and significantly more NCI in the hypoglossal nucleus than the other types. These findings extend previously described clinicopathological associations of FTLD-TDP subtypes and support the notion that FTLD-TDP subtypes may be distinct clinicopathologic disorders.
Figures


References
-
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Dachsel JC, Ross OA, Mata IF, et al. Lrrk2 G2019S substitution in frontotemporal lobar degeneration with ubiquitin-immunoreactive neuronal inclusions. Acta Neuropathol. 2007;113:601–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

