The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species
- PMID: 19457242
- PMCID: PMC2693113
- DOI: 10.1186/1746-4811-5-6
The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species
Abstract
Background: Plant genome sequencing has resulted in the identification of a large number of uncharacterized genes. To investigate these unknown gene functions, several transient transformation systems have been developed as quick and convenient alternatives to the lengthy transgenic assay. These transient assays include biolistic bombardment, protoplast transfection and Agrobacterium-mediated transient transformation, each having advantages and disadvantages depending on the research purposes.
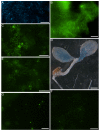
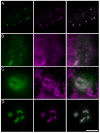
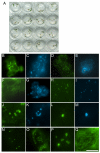
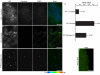
Results: We present a novel transient assay based on cocultivation of young Arabidopsis (Arabidopsis thaliana) seedlings with Agrobacterium tumefaciens in the presence of a surfactant which does not require any dedicated equipment and can be carried out within one week from sowing seeds to protein analysis. This Fast Agro-mediated Seedling Transformation (FAST) was used successfully to express a wide variety of constructs driven by different promoters in Arabidopsis seedling cotyledons (but not roots) in diverse genetic backgrounds. Localizations of three previously uncharacterized proteins were identified by cotransformation with fluorescent organelle markers. The FAST procedure requires minimal handling of seedlings and was also adaptable for use in 96-well plates. The high transformation efficiency of the FAST procedure enabled protein detection from eight transformed seedlings by immunoblotting. Protein-protein interaction, in this case HY5 homodimerization, was readily detected in FAST-treated seedlings with Förster resonance energy transfer and bimolecular fluorescence complementation techniques. Initial tests demonstrated that the FAST procedure can also be applied to other dicot and monocot species, including tobacco, tomato, rice and switchgrass.
Conclusion: The FAST system provides a rapid, efficient and economical assay of gene function in intact plants with minimal manual handling and without dedicated device. This method is potentially ideal for future automated high-throughput analysis.
Figures


References
-
- Christou P. Strategies for variety-independent genetic transformation of important cereals, legumes and woody species utilizing particle bombardment. Euphytica. 1995;85:13–27. doi: 10.1007/BF00023926. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

