CTLA4 blockade increases Th17 cells in patients with metastatic melanoma
- PMID: 19457253
- PMCID: PMC2697137
- DOI: 10.1186/1479-5876-7-35
CTLA4 blockade increases Th17 cells in patients with metastatic melanoma
Abstract
Background: Th17 cells are CD4+ cells that produce interleukin 17 (IL-17) and are potent inducers of tissue inflammation and autoimmunity. We studied the levels of this T cell subset in peripheral blood of patients treated with the anti-CTLA4 antibody tremelimumab since its major dose limiting toxicities are inflammatory and autoimmune in nature.
Methods: Peripheral blood mononuclear cells (PBMC) were collected before and after receiving tremelimumab within two clinical trials, one with tremelimumab alone (21 patients) and another together with autologous dendritic cells (DC) pulsed with the melanoma epitope MART-126-35 (6 patients). Cytokines were quantified directly in plasma from patients and after in vitro stimulation of PBMC. We also quantified IL-17 cytokine-producing cells by intracellular cytokine staining (ICS).
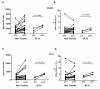
Results: There were no significant changes in 13 assayed cytokines, including IL-17, when analyzing plasma samples obtained from patients before and after administration of tremelimumab. However, when PBMC were activated in vitro, IL-17 cytokine in cell culture supernatant and Th17 cells, detected as IL-17-producing CD4 cells by ICS, significantly increased in post-dosing samples. There were no differences in the levels of Th17 cells between patients with or without an objective tumor response, but samples from patients with inflammatory and autoimmune toxicities during the first cycle of therapy had a significant increase in Th17 cells.
Conclusion: The anti-CTLA4 blocking antibody tremelimumab increases Th17 cells in peripheral blood of patients with metastatic melanoma. The relation between increases in Th17 cells and severe autoimmune toxicity after CTLA4 blockade may provide insights into the pathogenesis of anti-CTLA4-induced toxicities.
Trial registration: ClinicalTrials.gov NCT00471887.
Figures



References
-
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. - DOI - PMC - PubMed
-
- Ribas A, Hauschild A, Kefford R, Punt CA, Haanen JB, Marmol M, Garbe C, Gomez-Navarro J, Pavlov D, Marshall M. Phase III, Open-Label, Randomized, Comparative Study of Tremelimumab (CP-675,206) and Chemotherapy (Temozolomide or Dacarbazine) in Patients with Advanced Melanoma. J Clin Oncol. 2008;26:9011.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

