Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis
- PMID: 19457256
- PMCID: PMC2696456
- DOI: 10.1186/1471-2334-9-67
Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis
Abstract
Background: One of the challenges facing the tuberculosis (TB) control programmes in resource-limited settings is lack of rapid techniques for detection of drug resistant TB, particularly multi drug resistant tuberculosis (MDR TB). Results obtained with the conventional indirect susceptibility testing methods come too late to influence a timely decision on patient management. More rapid tests directly applied on sputum samples are needed. This study compared the sensitivity, specificity and time to results of four direct drug susceptibility testing tests with the conventional indirect testing for detection of resistance to rifampicin and isoniazid in M. tuberculosis. The four direct tests included two in-house phenotypic assays - Nitrate Reductase Assay (NRA) and Microscopic Observation Drug Susceptibility (MODS), and two commercially available tests - Genotype MTBDR and Genotype MTBDRplus (Hain Life Sciences, Nehren, Germany).
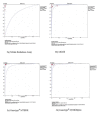
Methods: A literature review and meta-analysis of study reports was performed. The Meta-Disc software was used to analyse the reports and tests for sensitivity, specificity, and area under the summary receiver operating characteristic (sROC) curves. Heterogeneity in accuracy estimates was tested with the Spearman correlation coefficient and Chi-square.
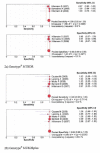
Results: Eighteen direct DST reports were analysed: NRA - 4, MODS- 6, Genotype MTBDR - 3 and Genotype MTBDRplus - 5. The pooled sensitivity and specificity for detection of resistance to rifampicin were 99% and 100% with NRA, 96% and 96% with MODS, 99% and 98% with Genotype MTBDR, and 99% and 99% with the new Genotype MTBDRplus, respectively. For isoniazid it was 94% and 100% for NRA, 92% and 96% for MODS, 71% and 100% for Genotype MTBDR, and 96% and 100% with the Genotype MTBDRplus, respectively. The area under the summary receiver operating characteristic (sROC) curves was in ranges of 0.98 to 1.00 for all the four tests. Molecular tests were completed in 1 - 2 days and also the phenotypic assays were much more rapid than conventional testing.
Conclusion: Direct testing of rifampicin and isoniazid resistance in M. tuberculosis was found to be highly sensitive and specific, and allows prompt detection of MDR TB.
Figures




References
-
- WHO. Global tuberculosis control 2008: surveillance, planning, financing. Geneva, Switzerland: World Health Organization; Publication no. WHO/HTM/TB/2008.393) 2008.
-
- WHO/IUATLD. Global Project on Anti-Tuberculosis Drug Resistance Surveillance: Anti-Tuberculosis drug resistance in the world, Report No.4, Annex 9. WHO/HTM/TB/2008.394 Geneva. 2008.
-
- Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

