Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow
- PMID: 19457866
- PMCID: PMC2707208
- DOI: 10.1074/jbc.M109.009886
Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow
Abstract
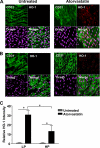
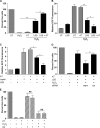
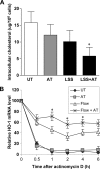
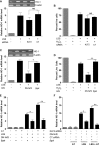
In addition to cholesterol-lowering properties, statins exhibit lipid-independent immunomodulatory, anti-inflammatory actions. However, high concentrations are typically required to induce these effects in vitro, raising questions concerning therapeutic relevance. We present evidence that endothelial cell sensitivity to statins depends upon shear stress. Using heme oxygenase-1 expression as a model, we demonstrate differential heme oxygenase-1 induction by atorvastatin in atheroresistant compared with atheroprone sites of the murine aorta. In vitro, exposure of human endothelial cells to laminar shear stress significantly reduced the statin concentration required to induce heme oxygenase-1 and protect against H(2)O(2)-mediated injury. Synergy was observed between laminar shear stress and atorvastatin, resulting in optimal expression of heme oxygenase-1 and resistance to oxidative stress, a response inhibited by heme oxygenase-1 small interfering RNA. Moreover, treatment of laminar shear stress-exposed endothelial cells resulted in a significant fall in intracellular cholesterol. Mechanistically, synergy required Akt phosphorylation, activation of Kruppel-like factor 2, NF-E2-related factor-2 (Nrf2), increased nitric-oxide synthase activity, and enhanced HO-1 mRNA stability. In contrast, heme oxygenase-1 induction by atorvastatin in endothelial cells exposed to oscillatory flow was markedly attenuated. We have identified a novel relationship between laminar shear stress and statins, demonstrating that atorvastatin-mediated heme oxygenase-1-dependent antioxidant effects are laminar shear stress-dependent, proving the principle that biomechanical signaling contributes significantly to endothelial responsiveness to pharmacological agents. Our findings suggest statin pleiotropy may be suboptimal at disturbed flow atherosusceptible sites, emphasizing the need for more specific therapeutic agents, such as those targeting Kruppel-like factor 2 or Nrf2.
Figures


References
-
- Jain M. K., Ridker P. M. (2005) Nat. Rev. Drug Discov. 4, 977–987 - PubMed
-
- Landmesser U., Bahlmann F., Mueller M., Spiekermann S., Kirchhoff N., Schulz S., Manes C., Fischer D., de Groot K., Fliser D., Fauler G., März W., Drexler H. (2005) Circulation 111, 2356–2363 - PubMed
-
- Mason J. C. (2003) Clin. Sci. 105, 251–266 - PubMed

