Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses
- PMID: 19457987
- PMCID: PMC2708648
- DOI: 10.1128/JVI.01434-08
Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses
Abstract
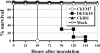
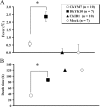
The Asian H5N1 highly pathogenic avian influenza (HPAI) viruses have been increasing in pathogenicity in diverse avian species since 1996 and are now widespread in Asian, European, and African countries. To better understand the basis of the increased pathogenicity of recent Asian H5N1 HPAI viruses in chickens, we compared the fevers and mean death times (MDTs) of chickens infected with the Asian H5N1 A/chicken/Yamaguchi/7/04 (CkYM7) strain with those infected with the H5N1 Duck/Yokohama/aq10/03 (DkYK10) strain, using a wireless thermosensor. Asian H5N1 CkYM7 caused peracute death in chickens before fever could be induced, whereas DkYK10 virus induced high fevers and had a long MDT. Real-time PCR analyses of cytokine mRNA expressions showed that CkYM7 quickly induced antiviral and proinflammatory cytokine mRNA expressions at 24 h postinfection (hpi) that suddenly decreased at 32 hpi. In contrast, these cytokine mRNA expressions increased at 24 hpi in the DkYK10 group, but decreased from 48 hpi onward to levels similar to those resulting from infection with the low-pathogenicity H5N2 A/chicken/Ibaraki/1/2004 strain. Sequential titrations of viruses in lungs, spleens, and kidneys demonstrated that CkYM7 replicated rapidly and efficiently in infected chickens and that the viral titers were more than twofold higher than those of DkYK10. CkYM7 preferentially and efficiently replicated in macrophages and vascular endothelial cells, while DkYK10 grew moderately in macrophages. These results indicate that the increased pathogenicity in chickens of the recent Asian H5N1 HPAI viruses may be associated with extremely rapid and high replication of the virus in macrophages and vascular endothelial cells, which resulted in disruption of the thermoregulation system and innate immune responses.
Figures



Similar articles
-
NP body domain and PB2 contribute to increased virulence of H5N1 highly pathogenic avian influenza viruses in chickens.J Virol. 2011 Feb;85(4):1834-46. doi: 10.1128/JVI.01648-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123376 Free PMC article.
-
Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses.Vet Res. 2014 Nov 28;45(1):118. doi: 10.1186/s13567-014-0118-3. Vet Res. 2014. PMID: 25431115 Free PMC article.
-
Emergence of avian influenza viruses with enhanced transcription activity by a single amino acid substitution in the nucleoprotein during replication in chicken brains.J Virol. 2011 Oct;85(19):10354-63. doi: 10.1128/JVI.00605-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795332 Free PMC article.
-
Immune responses to avian influenza viruses in chickens.Virology. 2025 Feb;603:110405. doi: 10.1016/j.virol.2025.110405. Epub 2025 Jan 16. Virology. 2025. PMID: 39837219 Review.
-
Role of miRNA in Highly Pathogenic H5 Avian Influenza Virus Infection: An Emphasis on Cellular and Chicken Models.Viruses. 2024 Jul 9;16(7):1102. doi: 10.3390/v16071102. Viruses. 2024. PMID: 39066264 Free PMC article. Review.
Cited by
-
NLRC5 Serves as a Pro-viral Factor During Influenza Virus Infection in Chicken Macrophages.Front Cell Infect Microbiol. 2020 May 19;10:230. doi: 10.3389/fcimb.2020.00230. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32509599 Free PMC article.
-
In vivo fitness associated with high virulence in a vertebrate virus is a complex trait regulated by host entry, replication, and shedding.J Virol. 2011 Apr;85(8):3959-67. doi: 10.1128/JVI.01891-10. Epub 2011 Feb 9. J Virol. 2011. PMID: 21307204 Free PMC article.
-
The avian and mammalian host range of highly pathogenic avian H5N1 influenza.Virus Res. 2013 Dec 5;178(1):3-11. doi: 10.1016/j.virusres.2013.09.004. Epub 2013 Sep 8. Virus Res. 2013. PMID: 24025480 Free PMC article. Review.
-
PA-X-associated early alleviation of the acute lung injury contributes to the attenuation of a highly pathogenic H5N1 avian influenza virus in mice.Med Microbiol Immunol. 2016 Aug;205(4):381-95. doi: 10.1007/s00430-016-0461-2. Epub 2016 Jun 11. Med Microbiol Immunol. 2016. PMID: 27289459 Free PMC article.
-
The Preferential Therapeutic Potential of Chlorella vulgaris against Aflatoxin-Induced Hepatic Injury in Quail.Toxins (Basel). 2022 Dec 1;14(12):843. doi: 10.3390/toxins14120843. Toxins (Basel). 2022. PMID: 36548739 Free PMC article.
References
-
- Alexander, D. J., G. Parsons, and R. J. Manvell. 1986. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol. 15647-662. - PubMed
-
- Amonsin, A., S. Payungporn, A. Theamboonlers, R. Thanawongnuwech, S. Suradhat, N. Pariyothorn, R. Tantilertcharoen, S. Damrongwantanapokin, C. Buranathai, A. Chaisingh, T. Songserm, and Y. Poovorawan. 2006. Genetic characterization of H5N1 influenza A viruses isolated from zoo tigers in Thailand. Virology 344480-491. - PubMed
-
- Brown, C. C., H. J. Olander, and D. A. Senne. 1992. A pathogenesis study of highly pathogenic avian influenza virus H5N2 in chickens, using immunohistochemistry. J. Comp. Pathol. 107341-348. - PubMed
-
- Chan, M. C., C. Y. Cheung, W. H. Chui, S. W. Tsao, J. M. Nicholls, Y. O. Chan, R. W. Chan, H. T. Long, L. L. Poon, Y. Guan, and J. S. Peiris. 2005. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 6135. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

