Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells
- PMID: 19458000
- PMCID: PMC2708622
- DOI: 10.1128/JVI.00182-09
Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells
Abstract
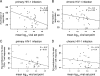
Primary HIV-1 infection (PHI) is marked by a flu-like syndrome and high levels of viremia that decrease to a viral set point with the first emergence of virus-specific CD8+ T-cell responses. Here, we investigated in a large cohort of 527 subjects the immunodominance pattern of the first virus-specific cytotoxic T-lymphocyte (CTL) responses developed during PHI in comparison to CTL responses in chronic infection and demonstrated a distinct relationship between the early virus-specific CTL responses and the viral set point, as well as the slope of CD4+ T-cell decline. CTL responses during PHI followed clear hierarchical immunodominance patterns that were lost during the transition to chronic infection. Importantly, the immunodominance patterns of human immunodeficiency virus type 1 (HIV-1)-specific CTL responses detected in primary, but not in chronic, HIV-1 infection were significantly associated with the subsequent set point of viral replication. Moreover, the preservation of the initial CD8+ T-cell immunodominance patterns from the acute into the chronic phase of infection was significantly associated with slower CD4+ T-cell decline. Taken together, these data show that the specificity of the initial CTL response to HIV is critical for the subsequent control of viremia and have important implications for the rational selection of antigens for future HIV-1 vaccines.
Figures

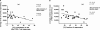
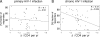
References
-
- Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 772081-2092. - PMC - PubMed
-
- Altfeld, M., E. T. Kalife, Y. Qi, H. Streeck, M. Lichterfeld, M. N. Johnston, N. Burgett, M. E. Swartz, A. Yang, G. Alter, X. G. Yu, A. Meier, J. K. Rockstroh, T. M. Allen, H. Jessen, E. S. Rosenberg, M. Carrington, and B. D. Walker. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med. 3e403. - PMC - PubMed
-
- Bashirova, A. A., G. Bleiber, Y. Qi, H. Hutcheson, T. Yamashita, R. C. Johnson, J. Cheng, G. Alter, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, M. May, F. Maldarelli, L. Jacobson, J. O'Brien, S. A. Telenti, and M. Carrington. 2006. Consistent effects of TSG101 genetic variability on multiple outcomes of exposure to human immunodeficiency virus type 1. J. Virol. 806757-6763. - PMC - PubMed
-
- Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 7511983-11991. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- P01 AI074415/AI/NIAID NIH HHS/United States
- R01 AI50429/AI/NIAID NIH HHS/United States
- U01 AI043638/AI/NIAID NIH HHS/United States
- N01 CO012400/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- AI43638/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States
- R01 AI050429/AI/NIAID NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- U01 AI052403/AI/NIAID NIH HHS/United States
- N01-CO-12400/CO/NCI NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

