Impaired quality of the hepatitis B virus (HBV)-specific T-cell response in human immunodeficiency virus type 1-HBV coinfection
- PMID: 19458009
- PMCID: PMC2708644
- DOI: 10.1128/JVI.00183-09
Impaired quality of the hepatitis B virus (HBV)-specific T-cell response in human immunodeficiency virus type 1-HBV coinfection
Abstract
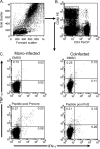
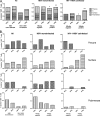
Hepatitis B virus (HBV)-specific T cells play a key role both in the control of HBV replication and in the pathogenesis of liver disease. Human immunodeficiency virus type 1 (HIV-1) coinfection and the presence or absence of HBV e (precore) antigen (HBeAg) significantly alter the natural history of chronic HBV infection. We examined the HBV-specific T-cell responses in treatment-naïve HBeAg-positive and HBeAg-negative HIV-1-HBV-coinfected (n = 24) and HBV-monoinfected (n = 39) Asian patients. Peripheral blood was stimulated with an overlapping peptide library for the whole HBV genome, and tumor necrosis factor alpha and gamma interferon cytokine expression in CD8+ T cells was measured by intracellular cytokine staining and flow cytometry. There was no difference in the overall magnitude of the HBV-specific T-cell responses, but the quality of the response was significantly impaired in HIV-1-HBV-coinfected patients compared with monoinfected patients. In coinfected patients, HBV-specific T cells rarely produced more than one cytokine and responded to fewer HBV proteins than in monoinfected patients. Overall, the frequency and quality of the HBV-specific T-cell responses increased with a higher CD4+ T-cell count (P = 0.018 and 0.032, respectively). There was no relationship between circulating HBV-specific T cells and liver damage as measured by activity and fibrosis scores, and the HBV-specific T-cell responses were not significantly different in patients with either HBeAg-positive or HBeAg-negative disease. The quality of the HBV-specific T-cell response is impaired in the setting of HIV-1-HBV coinfection and is related to the CD4+ T-cell count.
Figures
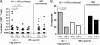
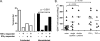
Similar articles
-
No increase in hepatitis B virus (HBV)-specific CD8+ T cells in patients with HIV-1-HBV coinfections following HBV-active highly active antiretroviral therapy.J Virol. 2010 Mar;84(6):2657-65. doi: 10.1128/JVI.02124-09. Epub 2010 Jan 6. J Virol. 2010. PMID: 20053751 Free PMC article.
-
Differential T-cell profiles determined by Hepatitis B surface antigen decrease among people with Human Immunodeficiency Virus /Hepatitis B Virus coinfection on treatment.J Transl Med. 2024 Oct 4;22(1):901. doi: 10.1186/s12967-024-05681-y. J Transl Med. 2024. PMID: 39367456 Free PMC article.
-
Hepatitis B virus (HBV)-specific T-cell responses to recombinant HBV core protein in patients with normal liver function and co-infected with chronic HBV and human immunodeficiency virus 1 (HIV-1).Virol J. 2013 Jul 12;10:232. doi: 10.1186/1743-422X-10-232. Virol J. 2013. PMID: 23849342 Free PMC article.
-
HBV Infection in HIV-Driven Immune Suppression.Viruses. 2019 Nov 19;11(11):1077. doi: 10.3390/v11111077. Viruses. 2019. PMID: 31752284 Free PMC article. Review.
-
Microbiota-Meditated Immunity Abnormalities Facilitate Hepatitis B Virus Co-Infection in People Living With HIV: A Review.Front Immunol. 2022 Jan 6;12:755890. doi: 10.3389/fimmu.2021.755890. eCollection 2021. Front Immunol. 2022. PMID: 35069530 Free PMC article. Review.
Cited by
-
HIV/HBV Co-Infections: Epidemiology, Natural History, and Treatment: A Review Article.Iran Red Crescent Med J. 2011 Dec;13(12):855-62. Epub 2011 Dec 1. Iran Red Crescent Med J. 2011. PMID: 22737429 Free PMC article.
-
No increase in hepatitis B virus (HBV)-specific CD8+ T cells in patients with HIV-1-HBV coinfections following HBV-active highly active antiretroviral therapy.J Virol. 2010 Mar;84(6):2657-65. doi: 10.1128/JVI.02124-09. Epub 2010 Jan 6. J Virol. 2010. PMID: 20053751 Free PMC article.
-
Hepatitis B virus surface antigen seroconversion in HIV-infected individual after pegylated interferon-alpha treatment: a case report.J Venom Anim Toxins Incl Trop Dis. 2013 Dec 10;19(1):31. doi: 10.1186/1678-9199-19-31. J Venom Anim Toxins Incl Trop Dis. 2013. PMID: 24325818 Free PMC article.
-
Sero-prevalence of HBV and associated risk factors among HIV positive individuals attending ART clinic at Mekelle hospital, Tigray, Northern Ethiopia.AIDS Res Ther. 2016 Feb 4;13:6. doi: 10.1186/s12981-016-0090-2. eCollection 2016. AIDS Res Ther. 2016. PMID: 26855663 Free PMC article.
-
Characteristics and outcomes of antiretroviral-treated HIV-HBV co-infected patients in Canada?BMC Infect Dis. 2019 Nov 21;19(1):982. doi: 10.1186/s12879-019-4617-8. BMC Infect Dis. 2019. PMID: 31752729 Free PMC article.
References
-
- Alatrakchi, N., C. S. Graham, Q. He, K. E. Sherman, and M. J. Koziel. 2005. CD8+ cell responses to hepatitis C virus (HCV) in the liver of persons with HCV-HIV coinfection versus HCV monoinfection. J. Infect. Dis. 191702-709. - PubMed
-
- Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. - PMC - PubMed
-
- Anonymous. 1994. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 2015-20. - PubMed
-
- Appay, V., D. C. Douek, and D. A. Price. 2008. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 14623-628. - PubMed
-
- Aye, T. T., A. Bartholomeusz, T. Shaw, S. Bowden, A. Breschkin, J. McMillan, P. Angus, and S. Locarnini. 1997. Hepatitis B virus polymerase mutations during antiviral therapy in a patient following liver transplantation. J. Hepatol. 261148-1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

