Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane-proximal external region of gp41
- PMID: 19458010
- PMCID: PMC2708617
- DOI: 10.1128/JVI.00656-09
Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane-proximal external region of gp41
Abstract
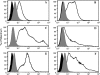
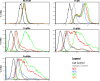
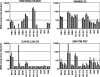
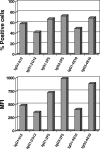
Receptors (FcgammaRs) for the constant region of immunoglobulin G (IgG) are an important link between humoral immunity and cellular immunity. To help define the role of FcgammaRs in determining the fate of human immunodeficiency virus type 1 (HIV-1) immune complexes, cDNAs for the four major human Fcgamma receptors (FcgammaRI, FcgammaRIIa, FcgammaRIIb, and FcgammaRIIIa) were stably expressed by lentiviral transduction in a cell line (TZM-bl) commonly used for standardized assessments of HIV-1 neutralization. Individual cell lines, each expressing a different FcgammaR, bound human IgG, as evidence that the physical properties of the receptors were preserved. In assays with a HIV-1 multisubtype panel, the neutralizing activities of two monoclonal antibodies (2F5 and 4E10) that target the membrane-proximal external region (MPER) of gp41 were potentiated by FcgammaRI and, to a lesser extent, by FcgammaRIIb. Moreover, the neutralizing activity of an HIV-1-positive plasma sample known to contain gp41 MPER-specific antibodies was potentiated by FcgammaRI. The neutralizing activities of monoclonal antibodies b12 and 2G12 and other HIV-1-positive plasma samples were rarely affected by any of the four FcgammaRs. Effects with gp41 MPER-specific antibodies were moderately stronger for IgG1 than for IgG3 and were ineffective for Fab. We conclude that FcgammaRI and FcgammaRIIb facilitate antibody-mediated neutralization of HIV-1 by a mechanism that is dependent on the Fc region, IgG subclass, and epitope specificity of antibody. The FcgammaR effects seen here suggests that the MPER of gp41 could have greater value for vaccines than previously recognized.
Figures

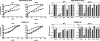

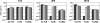
References
-
- Alam, S. M., M. McAdams, D. Boren, M. Rak, R. M. Scearce, F. Gao, Z. T. Camacho, D. Gewirth, G. Kelsoe, P. Chen, and B. F. Haynes. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 1784424-4435. - PMC - PubMed
-
- Barbato, G., E. Bianchi, P. Ingallinella, W. H. Hurni, M. D. Miller, G. Ciliberto, R. Cortese, R. Bazzo, J. W. Shiver, and A. Pessi. 2003. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J. Mol. Biol. 3301101-1115. - PubMed
-
- Barnes, N., A. L. Gavin, P. S. Tan, P. Mottram, F. Koentgen, and P. M. Hogarth. 2002. FcγRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity 16379-389. - PubMed
-
- Baum, L. L., K. J. Cassutt, K. Knigge, R. Khattri, J. Margolick, C. Rinaldo, C. A. Kleeberger, P. Nishanian, D. R. Henrard, and J. Phair. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 1572168-2173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

