Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides
- PMID: 19458039
- PMCID: PMC2690010
- DOI: 10.1073/pnas.0900288106
Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides
Abstract
Protein O-GlcNAcylation occurs in all animals and plants and is implicated in modulation of a wide range of cytosolic and nuclear protein functions, including gene silencing, nutrient and stress sensing, phosphorylation signaling, and diseases such as diabetes and Alzheimer's. The limiting factor impeding rapid progress in deciphering the biological functions of protein O-GlcNAcylation has been the inability to easily identify exact residues of modification. We describe a robust, high-sensitivity strategy able to assign O-GlcNAcylation sites of native modified peptides using electron transfer dissociation mass spectrometry. We have studied the murine postsynaptic density pseudoorganelle and report the assignment of 58 modification sites from a single experiment--significantly increasing the number of sites known in the literature. Components of several repressor complexes, such as NCoR1, polyhomeotic-like protein3, and EMSY, are modified. In addition, 28 O-GlcNAc sites were found on the protein Bassoon, effectively matching the number of phosphorylation sites reported previously on this protein. This finding suggests that on certain proteins, O-GlcNAcylation may be as extensive and important as phosphorylation in regulating protein function. Three of the newly discovered O-GlcNAc sites on Bassoon have previously been reported as phosphorylation sites, highlighting the interplay of the modifications. Surprisingly, several peptides with GlcNAc modifications on asparagines within the N-X-S/T consensus sequence were also observed from membrane protein extracellular domains. This powerful strategy fulfills a long-standing need in the biological community by facilitating modification site identifications that will accelerate understanding of the biological significance of this elusive regulatory posttranslational modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
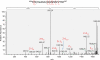



References
-
- Walsh CT. Posttranslational Modification of Proteins: Expanding Nature's Inventory. Englewood, CO: Roberts and Co.; 2006.
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. - PubMed
-
- Yang X, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

