A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma
- PMID: 19458046
- PMCID: PMC2684498
- DOI: 10.1073/pnas.0900591106
A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma
Abstract
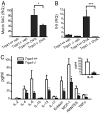
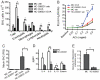
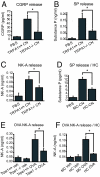
Asthma is an inflammatory disorder caused by airway exposures to allergens and chemical irritants. Studies focusing on immune, smooth muscle, and airway epithelial function revealed many aspects of the disease mechanism of asthma. However, the limited efficacies of immune-directed therapies suggest the involvement of additional mechanisms in asthmatic airway inflammation. TRPA1 is an irritant-sensing ion channel expressed in airway chemosensory nerves. TRPA1-activating stimuli such as cigarette smoke, chlorine, aldehydes, and scents are among the most prevalent triggers of asthma. Endogenous TRPA1 agonists, including reactive oxygen species and lipid peroxidation products, are potent drivers of allergen-induced airway inflammation in asthma. Here, we examined the role of TRPA1 in allergic asthma in the murine ovalbumin model. Strikingly, genetic ablation of TRPA1 inhibited allergen-induced leukocyte infiltration in the airways, reduced cytokine and mucus production, and almost completely abolished airway hyperreactivity to contractile stimuli. This phenotype is recapitulated by treatment of wild-type mice with HC-030031, a TRPA1 antagonist. HC-030031, when administered during airway allergen challenge, inhibited eosinophil infiltration and prevented the development of airway hyperreactivity. Trpa1(-/-) mice displayed deficiencies in chemically and allergen-induced neuropeptide release in the airways, providing a potential explanation for the impaired inflammatory response. Our data suggest that TRPA1 is a key integrator of interactions between the immune and nervous systems in the airways, driving asthmatic airway inflammation following inhaled allergen challenge. TRPA1 may represent a promising pharmacological target for the treatment of asthma and other allergic inflammatory conditions.
Conflict of interest statement
Conflict of interest statement: S.-E.J. is serving on the scientific advisory board of Hydra Biosciences, Cambridge, MA. Hydra Biosciences developed the TRPA1-antagonist, HC-030031, used in the present study. D.d.C., M.D., J.S.W., C.M.F., J.A.C., N.J.H., and M.M.M. are employees of Hydra Biosciences, and receive options.
Figures

References
-
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. - PubMed
-
- Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. - PubMed
-
- Cohn L, Elias JA, Chupp GL. Asthma: Mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. - PubMed
-
- Flood-Page P, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. - PubMed
-
- Undem BJ, Carr MJ. The role of nerves in asthma. Curr Allergy Asthma Rep. 2002;2:159–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

