Fibroblast growth factor receptor 1 promotes proliferation and survival via activation of the mitogen-activated protein kinase pathway in bladder cancer
- PMID: 19458078
- PMCID: PMC2737316
- DOI: 10.1158/0008-5472.CAN-08-2816
Fibroblast growth factor receptor 1 promotes proliferation and survival via activation of the mitogen-activated protein kinase pathway in bladder cancer
Abstract
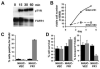
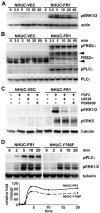
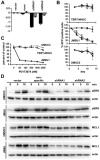
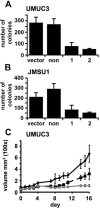
Fibroblast growth factor receptors (FGFR) play key roles in proliferation, differentiation, and tumorigenesis. Many urothelial carcinomas contain activating point mutations or increased expression of FGFR3. However, little is known about the role of other FGFRs. We examined FGFR expression in telomerase-immortalized normal human urothelial cells, urothelial carcinoma cell lines, and tumor samples and showed that FGFR1 expression is increased in a high proportion of cell lines and tumors independent of stage and grade. To determine the role of FGFR1 in low-stage bladder cancer, we overexpressed FGFR1 in telomerase-immortalized normal human urothelial cells and examined changes in proliferation and cell survival in response to FGF2. FGFR1 stimulation increased proliferation and reduced apoptosis. To elucidate the mechanistic basis for these alterations, we examined the signaling cascades activated by FGFR1. FRS2alpha and PLCgamma were activated in response to FGF2, leading to activation of the mitogen-activated protein kinase pathway. The level of mitogen-activated protein kinase activation correlated with the level of cyclin D1, MCL1, and phospho-BAD, which also correlated with FGFR-induced proliferation and survival. Knockdown of FGFR1 in urothelial carcinoma cell lines revealed differential FGFR1 dependence. JMSU1 cells were dependent on FGFR1 expression for survival but three other cell lines were not. Two cell lines (JMSU1 and UMUC3) were dependent on FGFR1 for growth in soft agar. Only one of the cell lines tested (UMUC3) was frankly tumorigenic; here, FGFR1 knockdown inhibited tumor growth. Our results indicate that FGFR1 has significant effects on urothelial cell phenotype and may represent a useful therapeutic target in some cases of urothelial carcinoma.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Cancer Research UK . UK Bladder Cancer Statistics. 2008.
-
- Knowles MA. Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese? Carcinogenesis. 2006;27:361–73. - PubMed
-
- Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. - PubMed
-
- Sangar VK, Ragavan N, Matanhelia SS, Watson MW, Blades RA. The economic consequences of prostate and bladder cancer in the UK. BJU Int. 2005;95:59–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

