Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices
- PMID: 19458085
- PMCID: PMC2740404
- DOI: 10.1074/jbc.M109.020362
Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices
Abstract
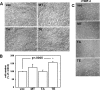
Increasing evidence suggests that the cytoplasmic tail of membrane type 1 matrix metalloproteinase (MT1-MMP) is subject to phosphorylation and that this modification may influence its enzymatic activity at the cell surface. In this study, phosphorylated MT1-MMP is detected using a phospho-specific antibody recognizing a protein kinase C consensus sequence (phospho-TXR), and a MT1-MMP tail peptide is phosphorylated by exogenous protein kinase C. To characterize the potential role of cytoplasmic residue Thr(567) in these processes, mutants that mimic a state of either constitutive (T567E) or defective phosphorylation (T567A) were expressed and analyzed for their functional effects on MT1-MMP activity and cellular behavior. Phospho-mimetic mutants of Thr(567) exhibit enhanced matrix invasion as well as more extensive growth within a three-dimensional type I collagen matrix. Together, these findings suggest that MT1-MMP surface action is regulated by phosphorylation at cytoplasmic tail residue Thr(567) and that this modification plays a critical role in processes that are linked to tumor progression.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

