CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset
- PMID: 19458112
- PMCID: PMC2705057
- DOI: 10.1104/pp.109.140269
CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset
Abstract
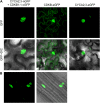
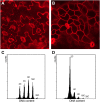
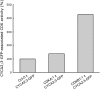
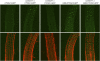
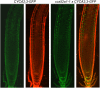
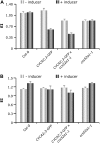
The mitosis-to-endocycle transition requires the controlled inactivation of M phase-associated cyclin-dependent kinase (CDK) activity. Previously, the B-type CDKB1;1 was identified as an important negative regulator of endocycle onset. Here, we demonstrate that CDKB1;1 copurifies and associates with the A2-type cyclin CYCA2;3. Coexpression of CYCA2;3 with CDKB1;1 triggered ectopic cell divisions and inhibited endoreduplication. Moreover, the enhanced endoreduplication phenotype observed after overexpression of a dominant-negative allele of CDKB1;1 could be partially complemented by CYCA2;3 co-overexpression, illustrating that both subunits unite in vivo to form a functional complex. CYCA2;3 protein stability was found to be controlled by CCS52A1, an activator of the anaphase-promoting complex. We conclude that CCS52A1 participates in endocycle onset by down-regulating CDKB1;1 activity through the destruction of CYCA2;3.
Figures
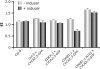


Similar articles
-
The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes.Plant Cell. 2005 Jun;17(6):1723-36. doi: 10.1105/tpc.105.032383. Epub 2005 Apr 29. Plant Cell. 2005. PMID: 15863515 Free PMC article.
-
Arabidopsis ULTRAVIOLET-B-INSENSITIVE4 maintains cell division activity by temporal inhibition of the anaphase-promoting complex/cyclosome.Plant Cell. 2011 Dec;23(12):4394-410. doi: 10.1105/tpc.111.091793. Epub 2011 Dec 13. Plant Cell. 2011. PMID: 22167059 Free PMC article.
-
UBIQUITIN-SPECIFIC PROTEASE14 Interacts with ULTRAVIOLET-B INSENSITIVE4 to Regulate Endoreduplication and Cell and Organ Growth in Arabidopsis.Plant Cell. 2016 May;28(5):1200-14. doi: 10.1105/tpc.16.00007. Epub 2016 Apr 20. Plant Cell. 2016. PMID: 27099260 Free PMC article.
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Established and novel Cdk/cyclin complexes regulating the cell cycle and development.Results Probl Cell Differ. 2011;53:365-89. doi: 10.1007/978-3-642-19065-0_16. Results Probl Cell Differ. 2011. PMID: 21630153 Review.
Cited by
-
Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis.Plant Cell. 2011 Sep;23(9):3260-75. doi: 10.1105/tpc.111.088583. Epub 2011 Sep 30. Plant Cell. 2011. PMID: 21963668 Free PMC article.
-
Phosphoproteomic analysis of distylous Turnera subulata identifies pathways related to endoreduplication that correlate with reciprocal herkogamy.Am J Bot. 2024 Dec;111(12):e16438. doi: 10.1002/ajb2.16438. Epub 2024 Nov 17. Am J Bot. 2024. PMID: 39551943 Free PMC article.
-
Cycling in a crowd: Coordination of plant cell division, growth, and cell fate.Plant Cell. 2022 Jan 20;34(1):193-208. doi: 10.1093/plcell/koab222. Plant Cell. 2022. PMID: 34498091 Free PMC article. Review.
-
Genome-Wide Identification, Characterization, and Transcriptomic Analysis of the Cyclin Gene Family in Brassica rapa.Int J Mol Sci. 2022 Nov 13;23(22):14017. doi: 10.3390/ijms232214017. Int J Mol Sci. 2022. PMID: 36430495 Free PMC article.
-
Transcriptional repression of the APC/C activator CCS52A1 promotes active termination of cell growth.EMBO J. 2012 Dec 12;31(24):4488-501. doi: 10.1038/emboj.2012.294. Epub 2012 Nov 9. EMBO J. 2012. PMID: 23143274 Free PMC article.
References
-
- Acquaviva C, Pines J (2006) The anaphase-promoting complex/cyclosome: APC/C. J Cell Sci 119 2401–2404 - PubMed
-
- Binné UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG Jr, Näär AM, Dyson NJ (2007) Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol 9 225–232 - PubMed
-
- Boudolf V, Vlieghe K, Beemster GTS, Magyar Z, Torres Acosta JA, Maes S, Van Der Schueren E, Inzé D, De Veylder L (2004. b) The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 16 2683–2692 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

