Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling
- PMID: 19458114
- PMCID: PMC2705021
- DOI: 10.1104/pp.109.139550
Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling
Abstract
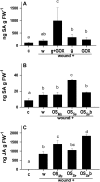
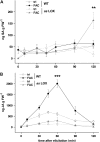
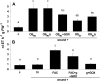
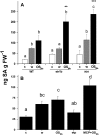
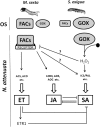
Salicylic acid (SA), jasmonic acid (JA), ethylene (ET), and their interactions mediate plant responses to pathogen and herbivore attack. JA-SA and JA-ET cross-signaling are well studied, but little is known about SA-ET cross-signaling in plant-herbivore interactions. When the specialist herbivore tobacco hornworm (Manduca sexta) attacks Nicotiana attenuata, rapid and transient JA and ET bursts are elicited without significantly altering wound-induced SA levels. In contrast, attack from the generalist beet armyworm (Spodoptera exigua) results in comparatively lower JA and ET bursts, but amplified SA bursts. These phytohormone responses are mimicked when the species' larval oral secretions (OS(Se) and OS(Ms)) are added to puncture wounds. Fatty acid-amino acid conjugates elicit the JA and ET bursts, but not the SA burst. OS(Se) had enhanced glucose oxidase activity (but not beta-glucosidase activity), which was sufficient to elicit the SA burst and attenuate the JA and ET levels. It is known that SA antagonizes JA; glucose oxidase activity and associated hydrogen peroxide also antagonizes the ET burst. We examined the OS(Ms)-elicited SA burst in plants impaired in their ability to elicit JA (antisense [as]-lox3) and ET (inverted repeat [ir]-aco) bursts and perceive ET (35s-etr1b) after fatty acid-amino acid conjugate elicitation, which revealed that both ET and JA bursts antagonize the SA burst. Treating wild-type plants with ethephone and 1-methylcyclopropane confirmed these results and demonstrated the central role of the ET burst in suppressing the OS(Ms)-elicited SA burst. By suppressing the SA burst, the ET burst likely facilitates unfettered JA-mediated defense activation in response to herbivores that otherwise would elicit SA.
Figures



References
-
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276 945–949
-
- Baldwin IT (1988) The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia 77 378–381 - PubMed
-
- Baldwin IT, Halitschke R, Kessler A, Schittko U (2001) Merging molecular and ecological approaches in plant-insect interactions. Curr Opin Plant Biol 4 351–358 - PubMed
-
- Baldwin IT, Zhang ZP, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA (1997) Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201 397–404
-
- Cipollini D, Enright S, Traw MB, Bergelson J (2004) Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol Ecol 13 1643–1653 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

