AtBXL1 encodes a bifunctional beta-D-xylosidase/alpha-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells
- PMID: 19458117
- PMCID: PMC2705025
- DOI: 10.1104/pp.109.138388
AtBXL1 encodes a bifunctional beta-D-xylosidase/alpha-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells
Abstract
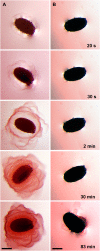
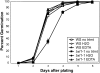
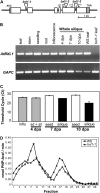
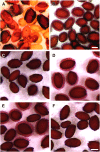
Following pollination, the epidermal cells of the Arabidopsis (Arabidopsis thaliana) ovule undergo a complex differentiation process that includes the synthesis and polar secretion of pectinaceous mucilage followed by the production of a secondary cell wall. Wetting of mature seeds leads to the rapid bursting of these mucilage secretory cells to release a hydrophilic gel that surrounds the seed and is believed to aid in seed hydration and germination. A novel mutant is identified where mucilage release is both patchy and slow and whose seeds display delayed germination. While developmental analysis of mutant seeds reveals no change in mucilage secretory cell morphology, changes in monosaccharide quantities are detected, suggesting the mucilage release defect results from altered mucilage composition. Plasmid rescue and cloning of the mutant locus revealed a T-DNA insertion in AtBXL1, which encodes a putative bifunctional beta-d-xylosidase/alpha-l-arabinofuranosidase that has been implicated as a beta-d-xylosidase acting during vascular development. Chemical and immunological analyses of mucilage extracted from bxl1 mutant seeds and antibody staining of developing seed coats reveal an increase in (1-->5)-linked arabinans, suggesting that BXL1 is acting as an alpha-l-arabinofuranosidase in the seed coat. This implication is supported by the ability to rescue mucilage release through treatment of bxl1 seeds with exogenous alpha-l-arabinofuranosidases. Together, these results suggest that trimming of rhamnogalacturonan I arabinan side chains is required for correct mucilage release and reveal a new role for BXL1 as an alpha-l-arabinofuranosidase acting in seed coat development.
Figures


References
-
- Alonso J, Stepanova AN, Leisse TJ, Kim CJ, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Bauer S, Vasu P, Mort AJ, Somerville CR (2005) Cloning, expression, and characterization of an oligoxyloglucan reducing end-specific xyloglucanbiohydrolase from Aspergillus nidulans. Carbohydr Res 340 2590–2597 - PubMed
-
- Beeckman T, De Rycke R, Viane R, Inzé D (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113 139–148
-
- Carpita N, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3 1–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

