Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice
- PMID: 19458127
- PMCID: PMC2724243
- DOI: 10.1152/ajprenal.00092.2009
Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice
Abstract
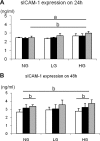
Hyperuricemia has recently been recognized to be a risk factor for nephropathy in the diabetic subject. We tested the hypothesis that lowering uric acid with a xanthine oxidase inhibitor might reduce renal injury in the diabetic mouse. Diabetic (db/db) mice were treated with allopurinol or no treatment for 8 wk. Serum uric acid, renal function, and histology were assessed at death. The direct effect of uric acid in human proximal tubular epithelial cells was also evaluated under normal or high glucose condition. We found that db/db mice developed hyperuricemia, albuminuria, mesangial matrix expansion, and mild tubulointerstitial disease. Allopurinol treatment significantly lowered uric acid levels, reduced albuminuria, and ameliorated tubulointerstitial injury, but it did not prevent mesangial expansion. The mechanism for protection was shown to be due to a reduction in inflammatory cells mediated by a reduction in ICAM-1 expression by tubular epithelial cells. Interestingly, allopurinol did not reduce oxidative stress in the kidney. An inflammatory role of uric acid on tubular cells was also confirmed by our in vitro evidence that uric acid directly induced ICAM-1 expression in the human proximal tubular cell. In conclusion, hyperuricemia has a pathogenic role in the mild tubulointerstitial injury associated with diabetic nephropathy but not glomerular damage in db/db mice. Lowering uric acid may reduce tubulointerstitial injury in diabetes.
Figures





References
-
- Afshari M, Larijani B, Rezaie A, Mojtahedi A, Zamani MJ, Astanehi-Asghari F, Mostafalou S, Hosseinnezhad A, Heshmat R, Abdollahi M. Ineffectiveness of allopurinol in reduction of oxidative stress in diabetic patients; a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother 58: 546–550, 2004. - PubMed
-
- Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31: 318–321, 2001. - PubMed
-
- Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51: 1118–1124, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

