Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways
- PMID: 19458143
- PMCID: PMC2724362
- DOI: 10.1152/jn.91003.2008
Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways
Abstract
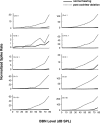
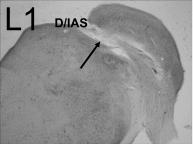
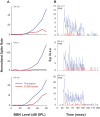
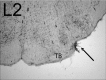
In the normal guinea pig, contralateral sound inhibits more than a third of ventral cochlear nucleus (VCN) neurons but excites <4% of these neurons. However, unilateral conductive hearing loss (CHL) and cochlear ablation (CA) result in a major enhancement of contralateral excitation. The response properties of the contralateral excitation produced by CHL and CA are similar, suggesting similar pathways are involved for both types of hearing loss. Here we used the neurotoxin melittin to test the hypothesis that this "compensatory" contralateral excitation is mediated either by direct glutamatergic CN-commissural projections or by cholinergic neurons of the olivocochlear bundle (OCB) that send collaterals to the VCN. Unit responses were recorded from the left VCN of anesthetized, unilaterally deafened guinea pigs (CHL via ossicular disruption, or CA via mechanical destruction). Neural responses were obtained with 16-channel electrodes to enable simultaneous data collection from a large number of single- and multiunits in response to ipsi- and contralateral tone burst and noise stimuli. Lesions of each pathway had differential effects on the contralateral excitation. We conclude that contralateral excitation has a fast and a slow component. The fast excitation is likely mediated by glutamatergic neurons located in medial regions of VCN that send their commissural axons to the other CN via the dorsal/intermediate acoustic striae. The slow component is likely mediated by the OCB collateral projections to the CN. Commissural neurons that leave the CN via the trapezoid body are an additional source of fast, contralateral excitation.
Figures







Similar articles
-
Effects of contralateral sound stimulation on unit activity of ventral cochlear nucleus neurons.Exp Brain Res. 2003 Dec;153(4):427-35. doi: 10.1007/s00221-003-1610-6. Epub 2003 Sep 5. Exp Brain Res. 2003. PMID: 12961054
-
Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss.J Neurophysiol. 2005 Dec;94(6):4234-43. doi: 10.1152/jn.00401.2005. Epub 2005 Aug 10. J Neurophysiol. 2005. PMID: 16093339
-
The commissural pathway and cochlear nucleus bushy neurons: an in vivo intracellular investigation.Brain Res. 2007 Feb 23;1134(1):113-21. doi: 10.1016/j.brainres.2006.11.058. Epub 2006 Dec 18. Brain Res. 2007. PMID: 17174943
-
The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus.Hear Res. 2011 Jun;276(1-2):61-9. doi: 10.1016/j.heares.2010.10.018. Epub 2010 Nov 4. Hear Res. 2011. PMID: 21056098 Free PMC article. Review.
-
Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei.Brain Res Bull. 2003 Jun 15;60(5-6):457-74. doi: 10.1016/s0361-9230(03)00050-9. Brain Res Bull. 2003. PMID: 12787867 Review.
Cited by
-
Mechanisms of Noise-Induced Tinnitus: Insights from Cellular Studies.Neuron. 2019 Jul 3;103(1):8-20. doi: 10.1016/j.neuron.2019.05.008. Neuron. 2019. PMID: 31271756 Free PMC article. Review.
-
Ringing ears: the neuroscience of tinnitus.J Neurosci. 2010 Nov 10;30(45):14972-9. doi: 10.1523/JNEUROSCI.4028-10.2010. J Neurosci. 2010. PMID: 21068300 Free PMC article. Review.
-
Functional connectivity networks in nonbothersome tinnitus.Otolaryngol Head Neck Surg. 2012 Nov;147(5):900-6. doi: 10.1177/0194599812451414. Epub 2012 Jun 21. Otolaryngol Head Neck Surg. 2012. PMID: 22722065 Free PMC article.
-
Tinnitus and underlying brain mechanisms.Curr Opin Otolaryngol Head Neck Surg. 2012 Oct;20(5):409-15. doi: 10.1097/MOO.0b013e3283577b81. Curr Opin Otolaryngol Head Neck Surg. 2012. PMID: 22931904 Free PMC article. Review.
-
Modulating central gain in tinnitus: changes in nitric oxide synthase in the ventral cochlear nucleus.Front Neurol. 2015 Mar 10;6:53. doi: 10.3389/fneur.2015.00053. eCollection 2015. Front Neurol. 2015. PMID: 25806021 Free PMC article.
References
-
- Adams JC Identification of cochlear nucleus projections by removal of HRP reaction product. Brain Res 177: 165–169, 1979a. - PubMed
-
- Adams JC Ascending projections to the inferior colliculus. J Comp Neurol 183: 519–538, 1979b. - PubMed
-
- Alibardi L Ultrastructural and immunocytochemical characterization of commissural neurons in the ventral cochlear nucleus of the rat. Anat Anz 180: 427–438, 1998. - PubMed
-
- Alibardi L Cytology, synaptology and immunocytochemistry of commissural neurons and their putative axonal terminals in the dorsal cochlear nucleus of the rat. Anat Anz 182: 207–220, 2000. - PubMed
-
- Alibardi L Mossy fibers in granule cell areas of the rat dorsal cochlear nucleus from intrinsic and extrinsic origin innervate unipolar brush cell glomeruli. J Submicrosc Cytol Pathol 36: 193–210, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

