Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment
- PMID: 19458190
- PMCID: PMC2710839
- DOI: 10.1091/mbc.e09-01-0071
Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment
Abstract
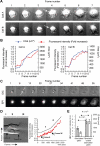
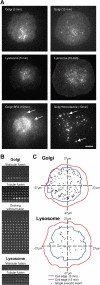
The role of plasma membrane (PM) area as a critical factor during cell motility is poorly understood, mainly due to an inability to precisely follow PM area dynamics. To address this fundamental question, we developed static and dynamic assays to follow exocytosis, endocytosis, and PM area changes during fibroblast spreading. Because the PM area cannot increase by stretch, spreading proceeds by the flattening of membrane folds and/or by the addition of new membrane. Using laser tweezers, we found that PM tension progressively decreases during spreading, suggesting the addition of new membrane. Next, we found that exocytosis increases the PM area by 40-60% during spreading. Reducing PM area reduced spread area, and, in a reciprocal manner, reducing spreadable area reduced PM area, indicating the interconnection between these two parameters. We observed that Golgi, lysosomes, and glycosylphosphatidylinositol-anchored protein vesicles are exocytosed during spreading, but endoplasmic reticulum and transferrin receptor-containing vesicles are not. Microtubule depolymerization blocks lysosome and Golgi exocytosis but not the exocytosis of glycosylphosphatidylinositol-anchored protein vesicles or PM area increase. Therefore, we suggest that fibroblasts are able to regulate about half of their original PM area by the addition of membrane via a glycosylphosphatidylinositol-anchored protein compartment.
Figures





Comment in
- Mol Biol Cell. 20:3169.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

