Scribble interacts with beta-catenin to localize synaptic vesicles to synapses
- PMID: 19458197
- PMCID: PMC2710836
- DOI: 10.1091/mbc.e08-12-1172
Scribble interacts with beta-catenin to localize synaptic vesicles to synapses
Abstract
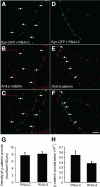
An understanding of how synaptic vesicles are recruited to and maintained at presynaptic compartments is required to discern the molecular mechanisms underlying presynaptic assembly and plasticity. We have previously demonstrated that cadherin-beta-catenin complexes cluster synaptic vesicles at presynaptic sites. Here we show that scribble interacts with the cadherin-beta-catenin complex to coordinate vesicle localization. Scribble and beta-catenin are colocalized at synapses and can be coimmunoprecipitated from neuronal lysates, indicating an interaction between scribble and beta-catenin at the synapse. Using an RNA interference approach, we demonstrate that scribble is important for the clustering of synaptic vesicles at synapses. Indeed, in scribble knockdown cells, there is a diffuse distribution of synaptic vesicles along the axon, and a deficit in vesicle recycling. Despite this, synapse number and the distribution of the presynaptic active zone protein, bassoon, remain unchanged. These effects largely phenocopy those observed after ablation of beta-catenin. In addition, we show that loss of beta-catenin disrupts scribble localization in primary neurons but that the localization of beta-catenin is not dependent on scribble. Our data supports a model by which scribble functions downstream of beta-catenin to cluster synaptic vesicles at developing synapses.
Figures





Similar articles
-
β-Pix modulates actin-mediated recruitment of synaptic vesicles to synapses.J Neurosci. 2011 Nov 23;31(47):17123-33. doi: 10.1523/JNEUROSCI.2359-11.2011. J Neurosci. 2011. PMID: 22114281 Free PMC article.
-
Postsynaptic Y654 dephosphorylation of β-catenin modulates presynaptic vesicle turnover through increased n-cadherin-mediated transsynaptic signaling.Dev Neurobiol. 2017 Jan;77(1):61-74. doi: 10.1002/dneu.22411. Epub 2016 Jul 8. Dev Neurobiol. 2017. PMID: 27328456
-
BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions.J Cell Biol. 2006 Jul 17;174(2):289-99. doi: 10.1083/jcb.200601087. Epub 2006 Jul 10. J Cell Biol. 2006. PMID: 16831887 Free PMC article.
-
Amyloid-β and Synaptic Vesicle Dynamics: A Cacophonic Orchestra.J Alzheimers Dis. 2019;72(1):1-14. doi: 10.3233/JAD-190771. J Alzheimers Dis. 2019. PMID: 31561377 Review.
-
Molecular organization and assembly of the presynaptic active zone of neurotransmitter release.Results Probl Cell Differ. 2006;43:49-68. doi: 10.1007/400_012. Results Probl Cell Differ. 2006. PMID: 17068967 Review.
Cited by
-
A role for WNT/β-catenin signaling in the neural mechanisms of behavior.J Neuroimmune Pharmacol. 2012 Dec;7(4):763-73. doi: 10.1007/s11481-012-9350-7. Epub 2012 Mar 15. J Neuroimmune Pharmacol. 2012. PMID: 22415718 Free PMC article. Review.
-
Sorting of cadherin-catenin-associated proteins into individual clusters.Proc Natl Acad Sci U S A. 2021 Jul 20;118(29):e2105550118. doi: 10.1073/pnas.2105550118. Proc Natl Acad Sci U S A. 2021. PMID: 34272290 Free PMC article.
-
Regulation of the DLG tumor suppressor by β-catenin.Int J Cancer. 2012 Nov 15;131(10):2223-33. doi: 10.1002/ijc.27519. Epub 2012 Mar 28. Int J Cancer. 2012. PMID: 22392736 Free PMC article.
-
NOS1AP associates with Scribble and regulates dendritic spine development.J Neurosci. 2010 Mar 31;30(13):4796-805. doi: 10.1523/JNEUROSCI.3726-09.2010. J Neurosci. 2010. PMID: 20357130 Free PMC article.
-
Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung.Dev Biol. 2013 Jan 15;373(2):267-80. doi: 10.1016/j.ydbio.2012.11.012. Epub 2012 Nov 27. Dev Biol. 2013. PMID: 23195221 Free PMC article.
References
-
- Ahmari S. E., Buchanan J., Smith S. J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 2000;3:445–451. - PubMed
-
- Audebert S., et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 2004;14:987–995. - PubMed
-
- Bozdagi O., Shan W., Tanaka H., Benson D. L., Huntley G. W. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

