Context-specific grasp movement representation in the macaque anterior intraparietal area
- PMID: 19458215
- PMCID: PMC6665886
- DOI: 10.1523/JNEUROSCI.5479-08.2009
Context-specific grasp movement representation in the macaque anterior intraparietal area
Abstract
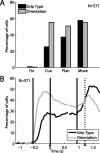
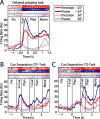
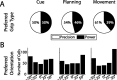
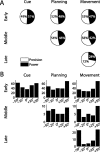
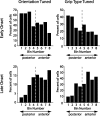
To perform grasping movements, the hand is shaped according to the form of the target object and the intended manipulation, which in turn depends on the context of the action. The anterior intraparietal cortex (AIP) is strongly involved in the sensorimotor transformation of grasping movements, but the extent to which it encodes context-specific information for hand grasping is unclear. To explore this issue, we recorded 571 single-units in AIP of two macaques during a delayed grasping task, in which animals were instructed by an external context cue (LED) to perform power or precision grips on a handle that was presented in various orientations. While 55% of the recorded neurons encoded the object orientation from the cue epoch on, the number of cells encoding the grip type increased from 25% during the cue epoch to 58% during movement execution. Furthermore, a classification of cells according to the time of their tuning onset revealed differences in the function and anatomical location of early- versus late-tuned cells. In a cue separation task, when the object was presented first, neurons representing power or precision grips were activated simultaneously until the actual grip type was instructed. In contrast, when the grasp type instruction was presented before the object, type information was only weakly represented in AIP, but was strongly encoded after the grasp target was revealed. We conclude that AIP encodes context specific hand grasping movements to perceived objects, but in the absence of a grasp target, the encoding of context information is weak.
Figures





Comment in
-
Simultaneous encoding of potential grasping movements in macaque anterior intraparietal area.J Neurosci. 2009 Sep 30;29(39):12031-2. doi: 10.1523/JNEUROSCI.3245-09.2009. J Neurosci. 2009. PMID: 19793960 Free PMC article. Review. No abstract available.
References
-
- Allport DA. Selection for action: some behavioral and neurophysiological considerations of attention and action. In: Heuer H, Sanders AF, editors. Perspectives on perception and action. Hillsdale, NJ: Erlbaum; 1987. pp. 395–419.
-
- Amemori K, Sawaguchi T. Rule-dependent shifting of sensorimotor representation in the primate prefrontal cortex. Eur J Neurosci. 2006;23:1895–1909. - PubMed
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. - PubMed
-
- Balint R. Seelenlähmung des “Schauens”, optische Ataxie, räumliche Störung der Aufmerksamkeit. Monatsschr Psychiatr Neurol. 1909;25:51–81.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
