Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone
- PMID: 19458216
- PMCID: PMC6665894
- DOI: 10.1523/JNEUROSCI.0708-09.2009
Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone
Abstract
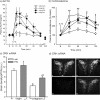
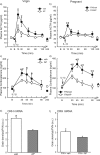
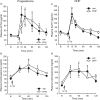
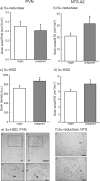
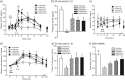
The hypothalamus-pituitary-adrenal (HPA) axis is the major neuroendocrine stress response system. Corticotropin-releasing hormone (CRH) neurons in the parvocellular paraventricular nucleus (pPVN) play a key role in coordinating responses of this system to stressors. The cytokine interleukin-1beta (IL-1beta), mimicking infection, robustly activates these CRH neurons via a noradrenergic input arising from the nucleus tractus solitarii (NTS). In late pregnancy, HPA axis responses to stressors, including IL-1beta, are attenuated by a central opioid mechanism that auto-inhibits noradrenaline release in the PVN. Here we show that the neuroactive progesterone metabolite allopregnanolone induces these changes in HPA responsiveness to IL-1beta in pregnancy. In late pregnancy, inhibition of 5alpha-reductase (an allopregnanolone-synthesizing enzyme) with finasteride restored HPA axis responses (rapidly increased pPVN CRH mRNA expression, ACTH, and corticosterone secretion) to IL-1beta. Conversely, allopregnanolone reduced HPA responses in virgin rats. In late pregnancy, activity of the allopregnanolone-synthesizing enzymes (5alpha-reductase and 3alpha-hydroxysteroid dehydrogenase) was increased in the hypothalamus as was mRNA expression in the NTS and PVN. Naloxone, an opioid antagonist, restores HPA axis responses to IL-1beta in pregnancy but had no additional effect after finasteride, indicating a causal connection between allopregnanolone and the endogenous opioid mechanism. Indeed, allopregnanolone induced opioid inhibition over HPA responses to IL-1beta in virgin rats. Furthermore, in virgin rats, allopregnanolone treatment increased, whereas in pregnant rats finasteride decreased proenkephalin-A mRNA expression in the NTS. Thus, in pregnancy, allopregnanolone induces opioid inhibition over HPA axis responses to immune challenge. This novel opioid-mediated mechanism of allopregnanolone action may alter regulation of other brain systems in pregnancy.
Figures
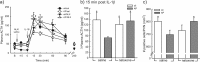

Similar articles
-
5α-Reduced neurosteroids sex-dependently reverse central prenatal programming of neuroendocrine stress responses in rats.J Neurosci. 2015 Jan 14;35(2):666-77. doi: 10.1523/JNEUROSCI.5104-13.2015. J Neurosci. 2015. PMID: 25589761 Free PMC article.
-
Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat.Stress. 2011 Jan;14(1):6-12. doi: 10.3109/10253890.2010.482628. Epub 2010 Jul 28. Stress. 2011. PMID: 20666638 Review.
-
Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats.J Neurosci. 2005 May 25;25(21):5117-26. doi: 10.1523/JNEUROSCI.0866-05.2005. J Neurosci. 2005. PMID: 15917452 Free PMC article.
-
Allopregnanolone and induction of endogenous opioid inhibition of oxytocin responses to immune stress in pregnant rats.J Neuroendocrinol. 2012 Apr;24(4):690-700. doi: 10.1111/j.1365-2826.2012.02295.x. J Neuroendocrinol. 2012. PMID: 22340139
-
Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: the neurosteroid opioid connection.J Physiol. 2008 Jan 15;586(2):369-75. doi: 10.1113/jphysiol.2007.146233. Epub 2007 Nov 8. J Physiol. 2008. PMID: 17991694 Free PMC article. Review.
Cited by
-
The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies.Biomolecules. 2022 Sep 8;12(9):1262. doi: 10.3390/biom12091262. Biomolecules. 2022. PMID: 36139100 Free PMC article. Review.
-
Prenatal Stress Alters Progestogens to Mediate Susceptibility to Sex-Typical, Stress-Sensitive Disorders, such as Drug Abuse: A Review.Front Psychiatry. 2011 Oct 17;2:52. doi: 10.3389/fpsyt.2011.00052. eCollection 2011. Front Psychiatry. 2011. PMID: 22022315 Free PMC article.
-
Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next?Front Endocrinol (Lausanne). 2020 May 14;11:236. doi: 10.3389/fendo.2020.00236. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32477260 Free PMC article. No abstract available.
-
Pubertal adversity alters chromatin dynamics and stress circuitry in the pregnant brain.Neuropsychopharmacology. 2020 Jul;45(8):1263-1271. doi: 10.1038/s41386-020-0634-y. Epub 2020 Feb 11. Neuropsychopharmacology. 2020. PMID: 32045935 Free PMC article.
-
Hormonal influences on neuroimmune responses in the CNS of females.Front Integr Neurosci. 2014 Jan 17;7:110. doi: 10.3389/fnint.2013.00110. eCollection 2013. Front Integr Neurosci. 2014. PMID: 24478642 Free PMC article. Review.
References
-
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. - PubMed
-
- Bali B, Kovács KJ. GABAergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Eur J Neurosci. 2003;18:1518–1526. - PubMed
-
- Beaulieu J, Champagne D, Drolet G. Enkephalin innervation of the paraventricular nucleus of the hypothalamus: distribution of fibers and origins of input. J Chem Neuroanat. 1996;10:79–92. - PubMed
-
- Bertics SJ, Bertics PJ, Clarke JL, Karavolas HJ. Distribution and ovarian control of progestin-metabolizing enzymes in various rat hypothalamic regions. J Steroid Biochem. 1987;26:321–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
