Synaptic scaling requires the GluR2 subunit of the AMPA receptor
- PMID: 19458219
- PMCID: PMC2714274
- DOI: 10.1523/JNEUROSCI.3753-08.2009
Synaptic scaling requires the GluR2 subunit of the AMPA receptor
Abstract
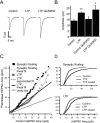
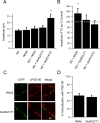
Two functionally distinct forms of synaptic plasticity, Hebbian long-term potentiation (LTP) and homeostatic synaptic scaling, are thought to cooperate to promote information storage and circuit refinement. Both arise through changes in the synaptic accumulation of AMPA receptors (AMPARs), but whether they use similar or distinct receptor-trafficking pathways is unknown. Here, we show that TTX-induced synaptic scaling in cultured visual cortical neurons leads to the insertion of GluR2-containing AMPARs at synapses. Similarly, visual deprivation with monocular TTX injections results in synaptic accumulation of GluR2-containing AMPARs. Unlike chemical LTP, synaptic scaling is blocked by a GluR2 C-tail peptide but not by a GluR1 C-tail peptide. Knockdown of endogenous GluR2 with an short hairpin RNA (shRNA) also blocks synaptic scaling but not chemical LTP. Scaling can be rescued with expression of exogenous GluR2 resistant to the shRNA, but a chimeric GluR2 subunit with the C-terminal domain swapped with the GluR1 C-terminal domain (GluR2/CT1) does not rescue synaptic scaling, indicating that regulatory sequences on the GluR2 C-tail are required for the accumulation of synaptic AMPARs during scaling. Together, our results suggest that synaptic scaling and LTP use different trafficking pathways, making these two forms of plasticity both functionally and molecularly distinct.
Figures

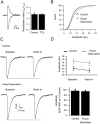
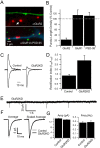
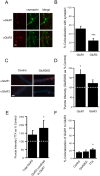
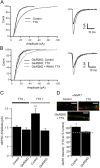
References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. - PubMed
-
- Akaneya Y. Activity regulates the expression of AMPA receptor subunit GluR4 in developing visual cortex. Eur J Neurosci. 2007;25:1641–1646. - PubMed
-
- Beretta F, Sala C, Saglietti L, Hirling H, Sheng M, Passafaro M. NSF interaction is important for direct insertion of GluR2 at synaptic sites. Mol Cell Neurosci. 2005;28:650–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
