Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses
- PMID: 19458233
- PMCID: PMC2943491
- DOI: 10.1523/JNEUROSCI.3829-08.2009
Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses
Abstract
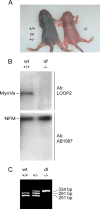
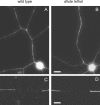
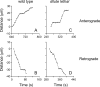
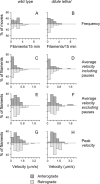
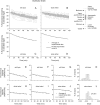
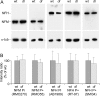
We investigated the axonal transport of neurofilaments in cultured neurons from two different strains of dilute lethal mice, which lack myosin Va. To analyze the motile behavior, we tracked the movement of green fluorescent protein (GFP)-tagged neurofilaments through naturally occurring gaps in the axonal neurofilament array of cultured superior cervical ganglion neurons from DLS/LeJ dilute lethal mice. Compared with wild-type controls, we observed no statistically significant difference in velocity or frequency of movement. To analyze the pausing behavior, we used a fluorescence photoactivation pulse-escape technique to measure the rate of departure of PAGFP (photoactivatable GFP)-tagged neurofilaments from photoactivated axonal segments in cultured dorsal root ganglion neurons from DLS/LeJ and dl20J dilute lethal mice. Compared with wild-type controls, we observed a 48% increase in the mean time for neurofilaments to depart the activated regions in neurons from DLS/LeJ mice (p < 0.001) and a 169% increase in neurons from dl20J mice (p < 0.0001). These data indicate that neurofilaments pause for more prolonged periods in the absence of myosin Va. We hypothesize that myosin Va is a short-range motor for neurofilaments and that it can function to enhance the efficiency of neurofilament transport in axons by delivering neurofilaments to their microtubule tracks, thereby reducing the duration of prolonged off-track pauses.
Figures



References
-
- Archer DR, Watson DF, Griffin JW. Phosphorylation-dependent immunoreactivity of neurofilaments and the rate of slow axonal transport in the central and peripheral axons of the rat dorsal root ganglion. J Neurochem. 1994;62:1119–1125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
