Inactivation of Ras by p120GAP via focal adhesion kinase dephosphorylation mediates RGMa-induced growth cone collapse
- PMID: 19458235
- PMCID: PMC6665895
- DOI: 10.1523/JNEUROSCI.0927-09.2009
Inactivation of Ras by p120GAP via focal adhesion kinase dephosphorylation mediates RGMa-induced growth cone collapse
Abstract
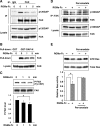
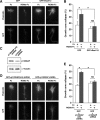
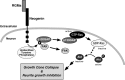
The repulsive guidance molecule RGMa performs several functions in the developing and adult CNSs. RGMa, through its receptor neogenin, induces growth cone collapse and neurite outgrowth inhibition. Here, we demonstrate that RGMa binding to neogenin leads to the inactivation of Ras, which is required for the RGMa-mediated repulsive function in cortical neurons. This signal transduction is mediated by the Ras-specific GTPase-activating protein (GAP) p120GAP. The SH2 domain of p120GAP interacts with focal adhesion kinase (FAK), which is phosphorylated at Tyr-397. When the cells are stimulated with RGMa, FAK undergoes dephosphorylation at Tyr-397 and is dissociated from p120GAP, and this dissociation is followed by an increase in the interaction between p120GAP and GTP-Ras. In addition, the knockdown of p120GAP prevents RGMa-induced growth cone collapse and neurite outgrowth inhibition. Furthermore, RGMa stimulation induces Akt inactivation through p120GAP, and the expression of the constitutively active Akt prevents RGMa-induced growth cone collapse. Thus, RGMa binding to neogenin regulates p120GAP activity through FAK Tyr-397 dephosphorylation, leading to the inactivation of Ras and its downstream effector Akt, and this signal transduction plays a role in the RGMa-mediated repulsive function.
Figures







Similar articles
-
Neogenin-RGMa signaling at the growth cone is bone morphogenetic protein-independent and involves RhoA, ROCK, and PKC.J Biol Chem. 2007 Jun 1;282(22):16423-33. doi: 10.1074/jbc.M610901200. Epub 2007 Mar 27. J Biol Chem. 2007. PMID: 17389603
-
Repulsive guidance molecule a suppresses seizures and mossy fiber sprouting via the FAK‑p120RasGAP‑Ras signaling pathway.Mol Med Rep. 2019 Apr;19(4):3255-3262. doi: 10.3892/mmr.2019.9951. Epub 2019 Feb 14. Mol Med Rep. 2019. PMID: 30816469
-
Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule.J Neurochem. 2008 Apr;105(1):113-26. doi: 10.1111/j.1471-4159.2007.05125.x. Epub 2007 Nov 12. J Neurochem. 2008. PMID: 18005226
-
Repulsive Guidance Molecule-a and Central Nervous System Diseases.Biomed Res Int. 2021 May 4;2021:5532116. doi: 10.1155/2021/5532116. eCollection 2021. Biomed Res Int. 2021. PMID: 33997000 Free PMC article. Review.
-
The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation.Curr Mol Med. 2003 Jun;3(4):313-24. doi: 10.2174/1566524033479744. Curr Mol Med. 2003. PMID: 12776987 Review.
Cited by
-
Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues.J Cell Sci. 2012 Jun 15;125(Pt 12):2918-29. doi: 10.1242/jcs.100107. Epub 2012 Mar 5. J Cell Sci. 2012. PMID: 22393238 Free PMC article.
-
Effective neuroprotection by ischemic postconditioning is associated with a decreased expression of RGMa and inflammation mediators in ischemic rats.Neurochem Res. 2013 Apr;38(4):815-25. doi: 10.1007/s11064-013-0984-5. Epub 2013 Feb 7. Neurochem Res. 2013. PMID: 23389659
-
MiR-552-3p promotes malignant progression of gallbladder carcinoma by reactivating the Akt/β-catenin signaling pathway due to inhibition of the tumor suppressor gene RGMA.Ann Transl Med. 2021 Sep;9(17):1374. doi: 10.21037/atm-21-2013. Ann Transl Med. 2021. PMID: 34733926 Free PMC article.
-
Regulation by noncoding RNAs of local translation, injury responses, and pain in the peripheral nervous system.Neurobiol Pain. 2023 Jan 24;13:100119. doi: 10.1016/j.ynpai.2023.100119. eCollection 2023 Jan-Jul. Neurobiol Pain. 2023. PMID: 36798094 Free PMC article.
-
RGMs: Structural Insights, Molecular Regulation, and Downstream Signaling.Trends Cell Biol. 2017 May;27(5):365-378. doi: 10.1016/j.tcb.2016.11.009. Epub 2016 Dec 19. Trends Cell Biol. 2017. PMID: 28007423 Free PMC article. Review.
References
-
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
-
- Brinks H, Conrad S, Vogt J, Oldekamp J, Sierra A, Deitinghoff L, Bechmann I, Alvarez-Bolado G, Heimrich B, Monnier PP, Mueller BK, Skutella T. The repulsive guidance molecule RGMa is involved in the formation of afferent connections in the dentate gyrus. J Neurosci. 2004;24:3862–3869. - PMC - PubMed
-
- Brouns MR, Matheson SF, Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat Cell Biol. 2001;3:361–367. - PubMed
-
- Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. - PubMed
-
- Conrad S, Genth H, Hofmann F, Just I, Skutella T. Neogenin-RGMa signaling at the growth cone is bone morphogenetic protein-independent and involves RhoA, ROCK, and PKC. J Biol Chem. 2007;7:784–792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
