Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth
- PMID: 19458237
- PMCID: PMC6665889
- DOI: 10.1523/JNEUROSCI.4361-08.2009
Lewis(x) and alpha2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth
Abstract
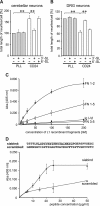
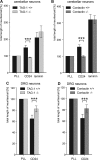
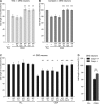
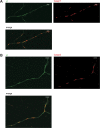
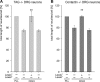
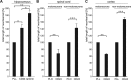
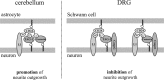
Although carbohydrates have been implicated in cell interactions in the nervous system, the molecular bases of their functions have remained largely obscure. Here, we show that promotion or inhibition of neurite outgrowth of cerebellar or dorsal root ganglion neurons, respectively, induced by the mucin-type adhesion molecule CD24 depends on alpha2,3-linked sialic acid and Lewis(x) present on glia-specific CD24 glycoforms. Alpha2,3-sialyl residues of CD24 bind to a structural motif in the first fibronectin type III domain of the adhesion molecule L1. Following the observation that the adhesion molecules TAG-1 and Contactin show sequence homologies with fucose-specific lectins, we obtained evidence that TAG-1 and Contactin mediate Lewis(x)-dependent CD24-induced effects on neurite outgrowth. Thus, L1, TAG-1, and Contactin function as lectin-like neuronal receptors. Their cis interactions with neighboring adhesion molecules, e.g., Caspr1 and Caspr2, and with their triggered signal transduction pathways elicit cell type-specific promotion or inhibition of neurite outgrowth induced by glial CD24 in a glycan-dependent trans interaction.
Figures




References
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Berglund EO, Murai KK, Fredette B, Sekerková G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated Contactin gene expression. Neuron. 1999;24:739–750. - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
