Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors
- PMID: 19458242
- PMCID: PMC2779219
- DOI: 10.1523/JNEUROSCI.1350-09.2009
Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors
Abstract
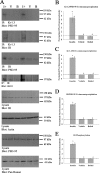
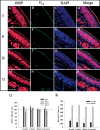
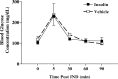
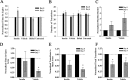
The role of insulin pathways in olfaction is of significant interest with the widespread pathology of diabetes mellitus and its associated metabolic and neuronal comorbidities. The insulin receptor (IR) kinase is expressed at high levels in the olfactory bulb, in which it suppresses a dominant Shaker ion channel (Kv1.3) via tyrosine phosphorylation of critical N- and C-terminal residues. We optimized a 7 d intranasal insulin delivery (IND) in awake mice to ascertain the biochemical and behavioral effects of insulin to this brain region, given that nasal sprays for insulin have been marketed notwithstanding our knowledge of the role of Kv1.3 in olfaction, metabolism, and axon targeting. IND evoked robust phosphorylation of Kv1.3, as well as increased channel protein-protein interactions with IR and postsynaptic density 95. IND-treated mice had an increased short- and long-term object memory recognition, increased anxiolytic behavior, and an increased odor discrimination using an odor habituation protocol but only moderate change in odor threshold using a two-choice paradigm. Unlike Kv1.3 gene-targeted deletion that alters metabolism, adiposity, and axonal targeting to defined olfactory glomeruli, suppression of Kv1.3 via IND had no effect on body weight nor the size and number of M72 glomeruli or the route of its sensory axon projections. There was no evidence of altered expression of sensory neurons in the epithelium. In mice made prediabetic via diet-induced obesity, IND was no longer effective in increasing long-term object memory recognition nor increasing anxiolytic behavior, suggesting state dependency or a degree of insulin resistance related to these behaviors.
Figures





Similar articles
-
Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice.Physiol Behav. 2017 May 15;174:104-113. doi: 10.1016/j.physbeh.2017.02.044. Epub 2017 Mar 2. Physiol Behav. 2017. PMID: 28259806 Free PMC article.
-
Electrophysiological and behavioral phenotype of insulin receptor defective mice.Physiol Behav. 2005 Oct 15;86(3):287-96. doi: 10.1016/j.physbeh.2005.08.024. Epub 2005 Sep 19. Physiol Behav. 2005. PMID: 16176826 Free PMC article.
-
Deletion of voltage-gated channel affects glomerular refinement and odorant receptor expression in the mouse olfactory system.J Comp Neurol. 2008 Jan 10;506(2):161-79. doi: 10.1002/cne.21540. J Comp Neurol. 2008. PMID: 18022950 Free PMC article.
-
The Olfactory Bulb: A Metabolic Sensor of Brain Insulin and Glucose Concentrations via a Voltage-Gated Potassium Channel.Results Probl Cell Differ. 2010;52:147-57. doi: 10.1007/978-3-642-14426-4_12. Results Probl Cell Differ. 2010. PMID: 20865378 Free PMC article. Review.
-
Engineering Aspects of Olfaction.In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 1. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 1. PMID: 26042329 Free Books & Documents. Review.
Cited by
-
High Fructose Diet inducing diabetes rapidly impacts olfactory epithelium and behavior in mice.Sci Rep. 2016 Sep 23;6:34011. doi: 10.1038/srep34011. Sci Rep. 2016. PMID: 27659313 Free PMC article.
-
Glucose sensitivity of mouse olfactory bulb neurons is conveyed by a voltage-gated potassium channel.J Physiol. 2013 May 15;591(10):2541-61. doi: 10.1113/jphysiol.2013.254086. Epub 2013 Mar 11. J Physiol. 2013. PMID: 23478133 Free PMC article.
-
Association of nerve conduction impairment and insulin resistance in children with obesity.Childs Nerv Syst. 2016 Nov;32(11):2219-2224. doi: 10.1007/s00381-016-3210-3. Epub 2016 Aug 9. Childs Nerv Syst. 2016. PMID: 27503137
-
Reduction in IGF1 mRNA in the Human Subependymal Zone During Aging.Aging Dis. 2019 Feb 1;10(1):197-204. doi: 10.14336/AD.2018.0317. eCollection 2019 Feb. Aging Dis. 2019. PMID: 30705779 Free PMC article.
-
Combined Effect of Fatty Diet and Cognitive Decline on Brain Metabolism, Food Intake, Body Weight, and Counteraction by Intranasal Insulin Therapy in 3×Tg Mice.Front Cell Neurosci. 2019 May 3;13:188. doi: 10.3389/fncel.2019.00188. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31130848 Free PMC article.
References
-
- Aimé P, Duchamp-Viret P, Chaput MA, Savigner A, Mahfouz M, Julliard AK. Fasting increases satiation decreases olfactory detection for a neutral odor in rats. Behav Brain Res. 2007;179:258–264. - PubMed
-
- Apelbaum AF, Chaput MA. Rats habituated to chronic feeding restriction show a smaller increase in olfactory bulb reactivity compared to newly fasted rats. Chem Senses. 2003;28:389–395. - PubMed
-
- Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–157. - PubMed
-
- Baly C, Aioun J, Badonnel K, Lacroix MC, Durieux D, Schlegel C, Salesse R, Caillol M. Leptin and its receptors are present in the rat olfactory mucosa and modulated by the nutritional status. Brain Res. 2007;1129:130–141. - PubMed
-
- Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
