Activation and repression domains within the promoter of the rat cathepsin L gene orchestrate sertoli cell-specific and stage-specific gene transcription in transgenic mice
- PMID: 19458314
- PMCID: PMC2731987
- DOI: 10.1095/biolreprod.109.075952
Activation and repression domains within the promoter of the rat cathepsin L gene orchestrate sertoli cell-specific and stage-specific gene transcription in transgenic mice
Abstract
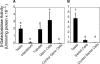
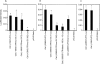
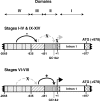
In murine testes, only Sertoli cells express the cathepsin L (Ctsl) gene, and this expression is restricted to stages V-VIII of the cycle. Our previous transgenic analysis of Tg (-2065/+977) demonstrated that this expression is regulated by a approximately 2-kb promoter. To begin to elucidate this regulation, we analyzed the in vivo expression of two new transgenes, Tg (-935/+977) and Tg (-451/+977). Tg (-935/+977) was expressed by Sertoli cells but, in contrast to Tg (-2065/+977), was expressed at all stages of the cycle, by spermatocytes, by the vascular endothelium, and by seven other organs. Tg (-451/+977) was not expressed by Sertoli cells but by spermatogenic cells and by the brain. Lack of expression of Tg (-451/+977) by Sertoli cells was not due to a lack of essential cis-acting elements. Transient transfection analysis of primary cultures of mature rat Sertoli cells demonstrated that in mature Sertoli cells, most of the activity of the Ctsl promoter is accounted for by one of two redundant upstream GC motifs and an Initiator that are within 100 bp of the transcription start site. We conclude that transcriptional repressors upstream from nucleotide -935 of the rat Ctsl gene restrict testicular expression of this gene to Sertoli cells at stages V-VIII. At these stages, transcriptional activators located between nucleotides -935 and -452 promote access of the transcriptional machinery to the two GC boxes and to the Initiator. Thus, upstream repressors and activators as well as cis-acting elements near the transcription start site control stage-specific Ctsl transcription by Sertoli cells.
Figures





Similar articles
-
A GC-box within the proximal promoter region of the rat cathepsin L gene activates transcription in Sertoli cells of sexually mature rats.Biol Reprod. 2003 May;68(5):1649-56. doi: 10.1095/biolreprod.102.012328. Epub 2002 Nov 27. Biol Reprod. 2003. PMID: 12606333
-
The cathepsin L first intron stimulates gene expression in rat sertoli cells.Biol Reprod. 2007 May;76(5):813-24. doi: 10.1095/biolreprod.106.057851. Epub 2007 Jan 17. Biol Reprod. 2007. PMID: 17229931
-
Distinct transcriptional mechanisms direct expression of the rat Dmrt1 promoter in sertoli cells and germ cells of transgenic mice.Biol Reprod. 2009 Jul;81(1):118-25. doi: 10.1095/biolreprod.108.072314. Epub 2009 Mar 4. Biol Reprod. 2009. PMID: 19264703 Free PMC article.
-
Male germ cells regulate transcription of the cathepsin l gene by rat Sertoli cells.Endocrinology. 2001 Jun;142(6):2318-27. doi: 10.1210/endo.142.6.8106. Endocrinology. 2001. PMID: 11356678
-
Germ cell-Sertoli cell interactions: regulation by germ cells of the stage-specific expression of CP-2/cathepsin L mRNA by Sertoli cells.Dev Genet. 1995;16(2):104-13. doi: 10.1002/dvg.1020160203. Dev Genet. 1995. PMID: 7736660
Cited by
-
The Regulation of Spermatogonial Stem Cells in an Adult Testis by Glial Cell Line-Derived Neurotrophic Factor.Front Endocrinol (Lausanne). 2022 Jun 3;13:896390. doi: 10.3389/fendo.2022.896390. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721702 Free PMC article. Review.
-
Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia.Biol Reprod. 2011 Oct;85(4):763-9. doi: 10.1095/biolreprod.110.087676. Epub 2011 Jun 8. Biol Reprod. 2011. PMID: 21653894 Free PMC article.
-
The "Glow"rious Sertoli and germ cells: mouse testis development visualized in multi-colors.Biol Reprod. 2011 Feb;84(2):201-4. doi: 10.1095/biolreprod.110.088856. Epub 2010 Oct 20. Biol Reprod. 2011. PMID: 20962250 Free PMC article.
-
Single-cell RNA-seq and pathological phenotype reveal the functional atlas and precise roles of Sox30 in testicular cell development and differentiation.Cell Death Dis. 2025 Feb 19;16(1):110. doi: 10.1038/s41419-025-07442-1. Cell Death Dis. 2025. PMID: 39971903 Free PMC article.
References
-
- Holdcraft RW, Braun RE.Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 2004; 131: 459–467.. - PubMed
-
- Wright WW, Smith L, Kerr C, Charron M.Mice that express enzymatically inactive cathepsin L exhibit abnormal spermatogenesis. Biol Reprod 2003; 68: 680–687.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

