The structure and function of G-protein-coupled receptors
- PMID: 19458711
- PMCID: PMC3967846
- DOI: 10.1038/nature08144
The structure and function of G-protein-coupled receptors
Abstract
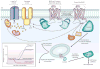
G-protein-coupled receptors (GPCRs) mediate most of our physiological responses to hormones, neurotransmitters and environmental stimulants, and so have great potential as therapeutic targets for a broad spectrum of diseases. They are also fascinating molecules from the perspective of membrane-protein structure and biology. Great progress has been made over the past three decades in understanding diverse GPCRs, from pharmacology to functional characterization in vivo. Recent high-resolution structural studies have provided insights into the molecular mechanisms of GPCR activation and constitutive activity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
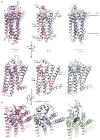
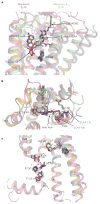

References
-
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. This paper provides a comprehensive analysis of sequence relationships between G-protein-coupled receptors in the human genome. - PubMed
-
- Hoffman BB, Lefkowitz RJ. Adrenergic receptors in the heart. Annu Rev Physiol. 1982;44:475–484. - PubMed
-
- Samama P, Pei G, Costa T, Cotecchia S, Lefkowitz RJ. Negative antagonists promote an inactive conformation of the beta 2-adrenergic receptor. Mol Pharmacol. 1994;45:390–394. - PubMed
-
- Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M. Inverse agonist activity of beta-adrenergic antagonists. Mol Pharmacol. 1994;45:490–499. - PubMed
-
- Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac beta(2)-adrenergic signal transduction. Circ Res. 1999;85:1092–1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

