Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation
- PMID: 19458789
- PMCID: PMC1828213
- DOI: 10.1039/b614610d
Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation
Abstract
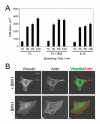
During cell adhesion to fibronectin there is a major reorganisation of the actin cytoskeleton and concomitant formation of adhesion complexes. Conflicting studies of adhesion receptors report that either integrin alone, or both integrin and syndecan-4 mediate the formation of vinculin-containing adhesions, and differences in these studies have been attributed to the density and conformational integrity of ligands used. We have endeavoured to resolve these issues by ELISA analysis of immobilised polypeptides, and found that ligands of both integrin alpha(5)beta(1) and syndecan-4 are necessary for focal adhesion formation under conditions of equivalent density of folded ligand. We also demonstrate that integrin and syndecan-4 play quite distinct roles in adhesion contact maturation and are not interchangeable. These results help us to understand how cells respond efficiently to changes in matrix environment, which should prove useful for developing approaches to aid wound healing.
Figures


References
-
- Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. J. Invest. Dermatol. 2003;121:695–705. - PubMed
-
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2805–10. - PMC - PubMed
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Tsuzuki S, Nakamura E, Kusugami K, Saito H, Muramatsu T. J. Biol. Chem. 2000;275:5249–52. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

