Review of the mechanism of processive actin filament elongation by formins
- PMID: 19459187
- PMCID: PMC2871153
- DOI: 10.1002/cm.20379
Review of the mechanism of processive actin filament elongation by formins
Abstract
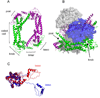
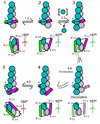
We review recent structural and biophysical studies of the mechanism of action of formins, proteins that direct the assembly of unbranched actin filaments for cytokinetic contractile rings and other cellular structures. Formins use free actin monomers to nucleate filaments and then remain bound to the barbed ends of these filaments as they elongate. In addition to variable regulatory domains, formins typically have formin homology 1 (FH1) and formin homology 2 (FH2) domains. FH1 domains have multiple binding sites for profilin, an abundant actin monomer binding protein. FH2 homodimers encircle the barbed end of a filament. Most FH2 domains inhibit actin filament elongation, but FH1 domains concentrate multiple profilin-actin complexes near the end of the filament. FH1 domains transfer actin very rapidly onto the barbed end of the filament, allowing elongation at rates that exceed the rate of elongation by the addition of free actin monomers diffusing in solution. Binding of actin to the end of the filament provides the energy for the highly processive movement of the FH2 as a filament adds thousands of actin subunits. These biophysical insights provide the context to understand how formins contribute to actin assembly in cells. Cell Motil. Cytoskeleton 2009. (c) 2009 Wiley-Liss, Inc.
Figures

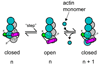

References
-
- Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276(4):2824–2830. - PubMed
-
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24(1):13–23. - PubMed
-
- Archer SJ, Vinson VK, Pollard TD, Torchia DA. Elucidation of the poly-L-proline binding site in Acanthamoeba profilin-I by NMR spectroscopy. FEBS Lett. 1994;337:145–151. - PubMed
-
- Blanchoin L, Pollard TD. Hydrolysis of ATP by polymerized actin depends on the bound divalent cation but not profilin. Biochemistry. 2002;41(2):597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

