Therapeutic antibodies: successes, limitations and hopes for the future
- PMID: 19459844
- PMCID: PMC2697811
- DOI: 10.1111/j.1476-5381.2009.00190.x
Therapeutic antibodies: successes, limitations and hopes for the future
Abstract
With more than 20 molecules in clinical use, monoclonal antibodies have finally come of age as therapeutics, generating a market value of $11 billion in 2004, expected to reach $26 billion by 2010. While delivering interesting results in the treatment of several major diseases including autoimmune, cardiovascular and infectious diseases, cancer and inflammation, clinical trials and research are generating a wealth of useful information, for instance about associations of clinical responses with Fc receptor polymorphisms and the infiltration and recruitment of effector cells into targeted tissues. Some functional limitations of therapeutic antibodies have come to light such as inadequate pharmacokinetics and tissue accessibility as well as impaired interactions with the immune system, and these deficiencies point to areas where additional research is needed. This review aims at giving an overview of the current state of the art and describes the most promising avenues that are being followed to create the next generation of antibody-based therapeutic agents.
Figures
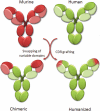
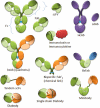
References
-
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. - PubMed
-
- Adams GP, Shaller CC, Dadachova E, Simmons HH, Horak EM, Tesfaye A, et al. A single treatment of yttrium-90-labeled CHX-A″-C6.5 diabody inhibits the growth of established human tumor xenografts in immunodeficient mice. Cancer Res. 2004;64:6200–6206. - PubMed
-
- Aires da Silva F, Santa-Marta M, Freitas-Vieira A, Mascarenhas P, Barahona I, Moniz-Pereira J, et al. Camelized rabbit-derived VH single-domain intrabodies against Vif strongly neutralize HIV-1 infectivity. J Mol Biol. 2004;340:525–542. - PubMed
-
- Alderson RF, Toki BE, Roberge M, Geng W, Basler J, Chin R, et al. Characterization of a CC49-based single-chain fragment-beta-lactamase fusion protein for antibody-directed enzyme prodrug therapy (ADEPT) Bioconjug Chem. 2006;17:410–418. - PubMed
-
- Amann M, Brischwein K, Lutterbuese P, Parr L, Petersen L, Lorenczewski G, et al. Therapeutic window of MuS110, a single-chain antibody construct bispecific for murine EpCAM and murine CD3. Cancer Res. 2008;68:143–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

