Quantification of mouse pulmonary cancer models by microcomputed tomography imaging
- PMID: 19459854
- PMCID: PMC11158256
- DOI: 10.1111/j.1349-7006.2009.01199.x
Quantification of mouse pulmonary cancer models by microcomputed tomography imaging
Abstract
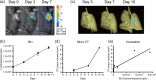
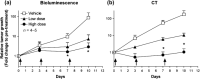
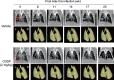
The advances in preclinical cancer models, including orthotopic implantation models or genetically engineered mouse models of cancer, enable pursuing the molecular mechanism of cancer disease that might mimic genetic and biological processes in humans. Lung cancer is the major cause of cancer deaths; therefore, the treatment and prevention of lung cancer are expected to be improved by a better understanding of the complex mechanism of disease. In this study, we have examined the quantification of two distinct mouse lung cancer models by utilizing imaging modalities for monitoring tumor progression and drug efficacy evaluation. The utility of microcomputed tomography (micro-CT) for real-time/non-invasive monitoring of lung cancer progression has been confirmed by combining bioluminescent imaging and histopathological analyses. Further, we have developed a more clinically relevant lung cancer model by utilizing K-ras(LSL-G12D)/p53(LSL-R270H) mutant mice. Using micro-CT imaging, we monitored the development and progression of solitary lung tumor in K-ras(LSL-G12D)/p53(LSL-R270H) mutant mouse, and further demonstrated tumor growth inhibition by anticancer drug treatment. These results clearly indicate that imaging-guided evaluation of more clinically relevant tumor models would improve the process of new drug discovery and increase the probability of success in subsequent clinical studies.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

