Synergistic effects of inward rectifier (I) and pacemaker (I) currents on the induction of bioengineered cardiac automaticity
- PMID: 19460073
- PMCID: PMC2739246
- DOI: 10.1111/j.1540-8167.2009.01475.x
Synergistic effects of inward rectifier (I) and pacemaker (I) currents on the induction of bioengineered cardiac automaticity
Abstract
Introduction: Normal heart rhythms originate in the sinoatrial node. HCN-encoded funny current (I(f)) and the Kir2-encoded inward rectifier (I(K1)) counteract each other by respectively oscillating and stabilizing the negative resting membrane potential, and controlling action potential firing. Therefore, I(K1) suppression and I(f) overexpression have been independently exploited to convert cardiomyocytes (CMs) into AP-firing bioartificial pacemakers. Although the 2 strategies have been largely assumed synergistic, their complementarity has not been investigated.
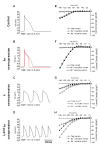
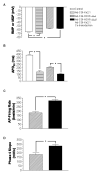
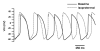
Methods and results: We explored the interrelationships of automaticity, I(f) and I(K1) by transducing single left ventricular (LV) CMs isolated from guinea pig hearts with the recombinant adenoviruses Ad-CMV-GFP-IRES-HCN1-AAA and/or Ad-CGI-Kir2.1 to mediate their current densities via a whole-cell patch clamp technique at 37 degrees C. Results showed that Ad-CGI-HCN1-AAA but not Ad-CGI-Kir2.1 transduction induced automaticity (181.1 +/- 13.1 bpm). Interestingly, Ad-CGI-HCN1-AAA/Ad-CGI-Kir2.1 cotransduction significantly promoted the induced firing frequency (320.0 +/- 15.8 bpm; P < 0.05). Correlation analysis revealed that the firing frequency, phase-4 slope and APD(90) of AP-firing LV CMs were correlated with I(f) (R(2) > 0.7) only when -2 >I(K1) >-4 pA/pF but not with I(K1) over the entire I(f) ranges examined (0.02 < R(2) < 0.4). Unlike I(f), I(K1) displayed correlation with neither the phase-4 slope (R(2)= 0.02) nor phase-4 length (R(2)= 0.04) when -2 > I(f) > -4 pA/pF. As anticipated, however, APD(90) was correlated with I(K1) (R(2)= 0.4).
Conclusion: We conclude that an optimal level of I(K1) maintains a voltage range for I(f) to operate most effectively during a dynamic cardiac cycle.
Figures



References
-
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–72. - PubMed
-
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. - PubMed
-
- Siu CW, Lieu DK, Li RA. HCN-encoded pacemaker channels: from physiology and biophysics to bioengineering. J Membr Biol. 2006;214(3):115–22. - PubMed
-
- Tse HF, Xue T, Lau CP, Siu CW, Wang K, Zhang QY, Tomaselli GF, Akar FG, Li RA. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN Channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation. 2006 Sep 5;114(10):1000–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

