Borrelia burgdorferi bb0426 encodes a 2'-deoxyribosyltransferase that plays a central role in purine salvage
- PMID: 19460093
- PMCID: PMC2764106
- DOI: 10.1111/j.1365-2958.2009.06740.x
Borrelia burgdorferi bb0426 encodes a 2'-deoxyribosyltransferase that plays a central role in purine salvage
Abstract
Borrelia burgdorferi is an obligate parasite with a limited genome that severely narrows its metabolic and biosynthetic capabilities. Thus survival of this spirochaete in an arthropod vector and mammalian host requires that it can scavenge amino acids, fatty acids and nucleosides from a blood meal or various host tissues. Additionally, the utilization of ribonucleotides for DNA synthesis is further complicated by the lack of a ribonucleotide reductase for the conversion of nucleoside-5'-diphosphates to deoxynucleosides-5'-diphosphates. The data presented here demonstrate that B. burgdorferi must rely on host-derived sources of purine bases, deoxypurines and deoxypyrimidines for the synthesis of DNA. However, if deoxyguanosine (dGuo) is limited in host tissue, the enzymatic activities of a 2'-deoxyribosyltransferase (DRTase, encoded by bb0426), IMP dehydrogenase (GuaB) and GMP synthase (GuaA) catalyse the multistep conversion of hypoxanthine (Hyp) to dGMP for DNA synthesis. This pathway provides additional biochemical flexibility for B. burgdorferi when it colonizes and infects different host tissues.
Figures



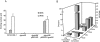
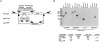

References
-
- Abbott JL, Newell JM, Lightcap CM, Olanich ME, Loughlin DT, Weller MA, et al. The effects of removing the GAT domain from E. coli GMP synthetase. Protein J. 2006;25:483–491. - PubMed
-
- Anand R, Kaminski PA, Ealick SE. Structures of purine 2′-deoxyribosyltransferase, substrate complexes, and the ribosylated enzyme intermediate at 2.0 A resolution. Biochemistry. 2004;43:2384–2393. - PubMed
-
- Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

