Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae
- PMID: 19460144
- PMCID: PMC2702294
- DOI: 10.1186/1472-6785-9-16
Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae
Abstract
Background: Ongoing lineage splitting within the African malaria mosquito Anopheles gambiae is compatible with ecological speciation, the evolution of reproductive isolation by divergent natural selection acting on two populations exploiting alternative resources. Divergence between two molecular forms (M and S) identified by fixed differences in rDNA, and characterized by marked, although incomplete, reproductive isolation is occurring in West and Central Africa. To elucidate the role that ecology and geography play in speciation, we carried out a countrywide analysis of An. gambiae M and S habitat requirements, and that of their chromosomal variants, across Burkina Faso.
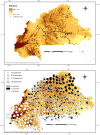
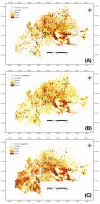
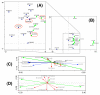
Results: Maps of relative abundance by geostatistical interpolators produced a distinct pattern of distribution: the M-form dominated in the northernmost arid zones, the S-form in the more humid southern regions. Maps of habitat suitability, quantified by Ecological Niche Factor Analysis based on 15 eco-geographical variables revealed less contrast among forms. M was peculiar as it occurred proportionally more in habitat of marginal quality. Measures of ecological niche breadth and overlap confirmed the mismatch between the fundamental and realized patterns of habitat occupation: forms segregated more than expected from the extent of divergence of their environmental envelope--a signature of niche expansion. Classification of chromosomal arm 2R karyotypes by multilocus genetic clustering identified two clusters loosely corresponding to molecular forms, with 'mismatches' representing admixed individuals due to shared ancestral polymorphism and/or residual hybridization. In multivariate ordination space, these karyotypes plotted in habitat of more marginal quality compared to non-admixed, 'typical', karyotypes. The distribution of 'typical' karyotypes along the main eco-climatic gradient followed a consistent pattern within and between forms, indicating an adaptive role of inversions at this geographical scale.
Conclusion: Ecological segregation between M and S is consistent with niche expansion into marginal habitats by chromosomal inversion variants during early lineage divergence; presumably, this process is promoted by inter-karyotype competition in the higher-quality core habitat. We propose that the appearance of favourable allelic combinations in other regions of suppressed recombination (e.g. pericentromeric portions defining speciation islands in An. gambiae) fosters development of reproductive isolation to protect linkage between separate chromosomal regions.
Figures



References
-
- Schluter D. Ecological causes of speciation. In: Howard DJ, Berlocher SH, editor. Endless Forms. Oxford: Oxford University Press; 1998. pp. 114–129.
-
- Rundle HD, Nosil P. Ecological speciation. Ecology Letters. 2005;8:336–352. doi: 10.1111/j.1461-0248.2004.00715.x. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

