In vitro activity of tigecycline in combination with various antimicrobials against multidrug resistant Acinetobacter baumannii
- PMID: 19460166
- PMCID: PMC2693502
- DOI: 10.1186/1476-0711-8-18
In vitro activity of tigecycline in combination with various antimicrobials against multidrug resistant Acinetobacter baumannii
Abstract
Background: Infections sustained by multidrug-resistant (MDR) and pan-resistant Acinetobacter baumannii have become a challenging problem in Intensive Care Units. Tigecycline provided new hope for the treatment of MDR A. baumannii infections, but isolates showing reduced susceptibility have emerged in many countries, further limiting the therapeutic options. Empirical combination therapy has become a common practice to treat patients infected with MDR A. baumannii, in spite of the limited microbiological and clinical evidence supporting its efficacy. Here, the in vitro interaction of tigecycline with seven commonly used anti-Acinetobacter drugs has been assessed.
Methods: Twenty-two MDR A. baumannii isolates from Intensive Care Unit (ICU) patients and two reference strains for the European clonal lineages I and II (including 3, 15 and 6 isolates that were resistant, intermediate and susceptible to tigecycline, respectively) were tested. Antimicrobial agents were: tigecycline, levofloxacin, piperacillin-tazobactam, amikacin, imipenem, rifampicin, ampicillin-sulbactam, and colistin. MICs were determined by the broth microdilution method. Antibiotic interactions were determined by chequerboard and time-kill assays. Only antibiotic combinations showing synergism or antagonism in both chequerboard and time-kill assays were accepted as authentic synergistic or antagonistic interactions, respectively.
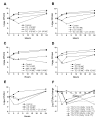
Results: Considering all antimicrobials in combination with tigecycline, chequerboard analysis showed 5.9% synergy, 85.7% indifference, and 8.3% antagonism. Tigecycline showed synergism with levofloxacin (4 strains; 16.6%), amikacin (2 strains; 8.3%), imipenem (2 strains; 8.3%) and colistin (2 strains; 8.3%). Antagonism was observed for the tigecycline/piperacillin-tazobactam combination (8 strains; 33.3%). Synergism was detected only among tigecycline non-susceptible strains. Time-kill assays confirmed the synergistic interaction between tigecycline and levofloxacin, amikacin, imipenem and colistin for 5 of 7 selected isolates. No antagonism was confirmed by time-kill assays.
Conclusion: This study demonstrates the in vitro synergistic activity of tigecycline in combination with colistin, levofloxacin, amikacin and imipenem against five tigecycline non-susceptible A. baumannii strains, opening the way to a more rationale clinical assessment of novel combination therapies to combat infections caused by MDR and pan-resistant A. baumannii.
Figures
Similar articles
-
In vitro time-kill studies of antimicrobial agents against blood isolates of imipenem-resistant Acinetobacter baumannii, including colistin- or tigecycline-resistant isolates.J Med Microbiol. 2012 Mar;61(Pt 3):353-360. doi: 10.1099/jmm.0.036939-0. Epub 2011 Oct 20. J Med Microbiol. 2012. PMID: 22016557
-
In Vitro Interactions of Antibiotic Combinations of Colistin, Tigecycline, and Doripenem Against Extensively Drug-Resistant and Multidrug-Resistant Acinetobacter baumannii.Ann Lab Med. 2016 Mar;36(2):124-30. doi: 10.3343/alm.2016.36.2.124. Ann Lab Med. 2016. PMID: 26709259 Free PMC article.
-
In vitro activity of doripenem in combination with various antimicrobials against multidrug-resistant Acinetobacter baumannii: possible options for the treatment of complicated infection.Microb Drug Resist. 2013 Oct;19(5):407-14. doi: 10.1089/mdr.2012.0250. Epub 2013 May 9. Microb Drug Resist. 2013. PMID: 23659601
-
In Vitro Activity of Various Antibiotics in Combination with Tigecycline Against Acinetobacter baumannii: A Systematic Review and Meta-Analysis.Microb Drug Resist. 2017 Dec;23(8):982-993. doi: 10.1089/mdr.2016.0279. Epub 2017 Apr 24. Microb Drug Resist. 2017. PMID: 28437233
-
Systematic Review of Antimicrobial Resistance of Clinical Acinetobacter baumannii Isolates in Iran: An Update.Microb Drug Resist. 2017 Sep;23(6):744-756. doi: 10.1089/mdr.2016.0118. Epub 2017 Jan 13. Microb Drug Resist. 2017. PMID: 28085571
Cited by
-
Antimicrobial Effects of Minocycline, Tigecycline, Ciprofloxacin, and Levofloxacin against Elizabethkingia anophelis Using In Vitro Time-Kill Assays and In Vivo Zebrafish Animal Models.Antibiotics (Basel). 2021 Mar 10;10(3):285. doi: 10.3390/antibiotics10030285. Antibiotics (Basel). 2021. PMID: 33801839 Free PMC article.
-
Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii.Antibiotics (Basel). 2021 Nov 3;10(11):1344. doi: 10.3390/antibiotics10111344. Antibiotics (Basel). 2021. PMID: 34827282 Free PMC article. Review.
-
Acinetobacter baumannii Resistance: A Real Challenge for Clinicians.Antibiotics (Basel). 2020 Apr 23;9(4):205. doi: 10.3390/antibiotics9040205. Antibiotics (Basel). 2020. PMID: 32340386 Free PMC article. Review.
-
Evaluation of the synergistic effect of a combination of colistin and tigecycline against multidrug-resistant Acinetobacterbaumannii.Pak J Med Sci. 2017 Mar-Apr;33(2):393-397. doi: 10.12669/pjms.332.11933. Pak J Med Sci. 2017. PMID: 28523044 Free PMC article.
-
Treatment for patients with multidrug resistant Acinetobacter baumannii pulmonary infection.Exp Ther Med. 2016 Apr;11(4):1345-1347. doi: 10.3892/etm.2016.3051. Epub 2016 Feb 8. Exp Ther Med. 2016. PMID: 27073447 Free PMC article.
References
-
- Nemec A, Krízová L, Maixnerová M, Diancourt L, Reijden TJ van der, Brisse S, Broek P van den, Dijkshoorn L. Emergence of carbapenem resistance in Acinetobacter baumannii in the Czech Republic is associated with the spread of multidrug-resistant strains of European clone II. J Antimicrob Chemother. 2008;62:484–489. doi: 10.1093/jac/dkn205. - DOI - PubMed
-
- Coelho JM, Turton JF, Kaufmann ME, Glover J, Woodford N, Warner M, Palepou MF, Pike R, Pitt TL, Patel BC, Livermore DM. Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J Clin Microbiol. 2006;44:3623–3627. doi: 10.1128/JCM.00699-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

