Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report
- PMID: 19460188
- PMCID: PMC5886713
- DOI: 10.1017/S0033291709006011
Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report
Abstract
Background: Many patients with major depressive disorder (MDD) who experience full symptomatic remission after antidepressant treatment still have residual depressive symptoms. We describe the types and frequency of residual depressive symptoms and their relationship to subsequent depressive relapse after treatment with citalopram in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.
Method: Participants in primary (n=18) and psychiatric (n=23) practice settings were openly treated with citalopram using measurement-based care for up to 14 weeks and follow-up for up to 1 year. We assessed 943 (32.8% of 2876) participants who met criteria for remission to determine the proportions with individual residual symptoms and any of the nine DSM-IV criterion symptom domains to define a major depressive episode. At each visit, the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR16) and the self-report Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale were used to assessed depressive symptoms and side-effects respectively.
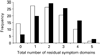
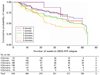
Results: More than 90% of remitters had at least one residual depressive symptom (median=3). The most common were weight increase (71.3%) and mid-nocturnal insomnia (54.9%). The most common residual symptom domains were sleep disturbance (71.7%) and appetite/weight disturbance (35.9%). Those who remitted before 6 weeks had fewer residual symptoms at study exit than did later remitters. Residual sleep disturbance did not predict relapse during follow-up. Having a greater number of residual symptom domains was associated with a higher probability of relapse.
Conclusions: Patients with remission of MDD after treatment with citalopram continue to experience selected residual depressive symptoms, which increase the risk of relapse.
Trial registration: ClinicalTrials.gov NCT00021528.
Figures

References
-
- Bockting C, Spinhoven P, Koeter M, Wouters L, Schene A Depression Evaluation Longitudinal Therapy Assessment (DELTA) Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. Journal of Clinical Psychiatry. 2006;67:747–755. - PubMed
-
- Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. Journal of Clinical Psychiatry. 2007;68:254–260. - PubMed
-
- DeBattista C, Doghramji K, Menza M, Rosenthal M, Fieve R. Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. Journal of Clinical Psychiatry. 2003;64:1057–1064. - PubMed
-
- Fava G, Ruini C, Belaise C. The concept of recovery in major depression. Psychological Medicine. 2007;37:307–317. - PubMed
-
- Fava GA, Fabbri S, Sonino N. Residual symptoms in depression: an emerging therapeutic target. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:1019–1027. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

