Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity
- PMID: 19460945
- PMCID: PMC2712435
- DOI: 10.1124/dmd.109.027565
Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity
Abstract
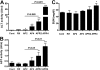
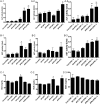
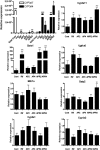
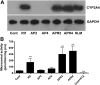
Acetaminophen (APAP) is safe at therapeutic levels but causes hepatotoxicity via N-acetyl-p-benzoquinone imine-induced oxidative stress upon overdose. To determine the effect of human (h) pregnane X receptor (PXR) activation and CYP3A4 induction on APAP-induced hepatotoxicity, mice humanized for PXR and CYP3A4 (TgCYP3A4/hPXR) were treated with APAP and rifampicin. Human PXR activation and CYP3A4 induction enhanced APAP-induced hepatotoxicity as revealed by hepatic alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities elevated in serum, and hepatic necrosis after coadministration of rifampicin and APAP, compared with APAP administration alone. In contrast, hPXR mice, wild-type mice, and Pxr-null mice exhibited significantly lower ALT/AST levels compared with TgCYP3A4/hPXR mice after APAP administration. Toxicity was coincident with depletion of hepatic glutathione and increased production of hydrogen peroxide, suggesting increased oxidative stress upon hPXR activation. Moreover, mRNA analysis demonstrated that CYP3A4 and other PXR target genes were significantly induced by rifampicin treatment. Urinary metabolomic analysis indicated that cysteine-APAP and its metabolite S-(5-acetylamino-2-hydroxyphenyl)mercaptopyruvic acid were the major contributors to the toxic phenotype. Quantification of plasma APAP metabolites indicated that the APAP dimer formed coincident with increased oxidative stress. In addition, serum metabolomics revealed reduction of lysophosphatidylcholine in the APAP-treated groups. These findings demonstrated that human PXR is involved in regulation of APAP-induced toxicity through CYP3A4-mediated hepatic metabolism of APAP in the presence of PXR ligands.
Figures



References
-
- Afonso V, Champy R, Mitrovic D, Collin P, and Lomri A (2007) Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74 324–329. - PubMed
-
- Bijlsma S, Bobeldijk I, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, van Ommen B, and Smilde AK (2006) Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem 78 567–574. - PubMed
-
- Carlquist JF, Muhlestein JB, and Anderson JL (2007) Lipoprotein-associated phospholipase A2: a new biomarker for cardiovascular risk assessment and potential therapeutic target. Expert Rev Mol Diagn 7 511–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

