ATP-citrate lyase links cellular metabolism to histone acetylation
- PMID: 19461003
- PMCID: PMC2746744
- DOI: 10.1126/science.1164097
ATP-citrate lyase links cellular metabolism to histone acetylation
Abstract
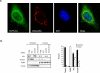
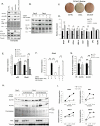
Histone acetylation in single-cell eukaryotes relies on acetyl coenzyme A (acetyl-CoA) synthetase enzymes that use acetate to produce acetyl-CoA. Metazoans, however, use glucose as their main carbon source and have exposure only to low concentrations of extracellular acetate. We have shown that histone acetylation in mammalian cells is dependent on adenosine triphosphate (ATP)-citrate lyase (ACL), the enzyme that converts glucose-derived citrate into acetyl-CoA. We found that ACL is required for increases in histone acetylation in response to growth factor stimulation and during differentiation, and that glucose availability can affect histone acetylation in an ACL-dependent manner. Together, these findings suggest that ACL activity is required to link growth factor-induced increases in nutrient metabolism to the regulation of histone acetylation and gene expression.
Figures


Comment in
-
Biochemistry. A glucose-to-gene link.Science. 2009 May 22;324(5930):1021-2. doi: 10.1126/science.1174665. Science. 2009. PMID: 19460991 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

