Coatomer-dependent protein delivery to lipid droplets
- PMID: 19461073
- PMCID: PMC2684835
- DOI: 10.1242/jcs.045849
Coatomer-dependent protein delivery to lipid droplets
Abstract
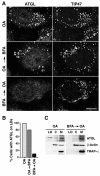
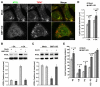
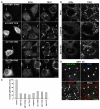
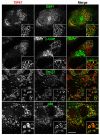
Lipid droplets (LDs) are cytoplasmic organelles that store neutral lipids for use as an energy supply in times of nutrient deprivation and for membrane assembly. Misregulation of LD function leads to many human diseases, including lipodystrophy, obesity and neutral lipid storage disorders. A number of proteins have been shown to localize to the surface of lipid droplets, including lipases such as adipose triglyceride lipase (ATGL) and the PAT-domain proteins ADRP (adipophilin) and TIP47, but the mechanism by which they are targeted to LDs is not known. Here we demonstrate that ATGL and ADRP, but not TIP47, are delivered to LDs by a pathway mediated by the COPI and COPII coatomer proteins and their corresponding regulators.
Figures



References
-
- Altan-Bonnet, N., Sougrat, R. and Lippincott-Schwartz, J. (2004). Molecular basis for Golgi maintenance and biogenesis. Curr. Opin. Cell Biol. 16, 364-372. - PubMed
-
- Arner, P. and Langin, D. (2007). The role of neutral lipases in human adipose tissue lipolysis. Curr. Opin. Lipidol. 18, 246-250. - PubMed
-
- Bartz, R., Zehmer, J. K., Zhu, M., Chen, Y., Serrero, G., Zhao, Y. and Liu, P. (2007). Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 6, 3256-3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

