The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response
- PMID: 19461119
- PMCID: PMC2781320
- DOI: 10.1194/jlr.M900216-JLR200
The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response
Abstract
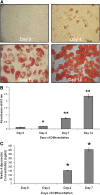
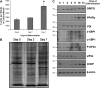
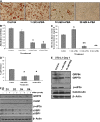
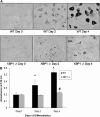
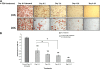
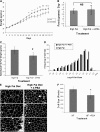
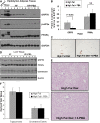
Recent studies have shown a link between obesity and endoplasmic reticulum (ER) stress. Perturbations in ER homeostasis cause ER stress and activation of the unfolded protein response (UPR). Adipocyte differentiation contributes to weight gain, and we have shown that markers of ER stress/UPR activation, including GRP78, phospho-eIF2, and spliced XBP1, are upregulated during adipogenesis. Given these findings, the objective of this study was to determine whether attenuation of UPR activation by the chemical chaperone 4-phenylbutyrate (4-PBA) inhibits adipogenesis. Exposure of 3T3-L1 preadipocytes to 4-PBA in the presence of differentiation media decreased expression of ER stress markers. Concomitant with the suppression of UPR activation, 4-PBA resulted in attenuation of adipogenesis as measured by lipid accumulation and adiponectin secretion. Consistent with these in vitro findings, female C57BL/6 mice fed a high-fat diet supplemented with 4-PBA showed a significant reduction in weight gain and had reduced fat pad mass, as compared with the high-fat diet alone group. Furthermore, 4-PBA supplementation decreased GRP78 expression in the adipose tissue and lowered plasma triglyceride, glucose, leptin, and adiponectin levels without altering food intake. Taken together, these results suggest that UPR activation contributes to adipogenesis and that blocking its activation with 4-PBA prevents adipocyte differentiation and weight gain in mice.
Figures
References
-
- Bray G. A.2004. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 89: 2583–2589 - PubMed
-
- Kosti R. I., Panagiotakos D. B. 2006. The epidemic of obesity in children and adolescents in the world. Cent. Eur. J. Public Health. 14: 151–159 - PubMed
-
- Gregoire F. M., Smas C. M., Sul H. S. 1998. Understanding adipocyte differentiation. Physiol. Rev. 78: 783–809 - PubMed
-
- Erol A.2005. Adipocyte insensitivity syndromes–novel approach to nutritional metabolic problems including obesity and obesity related disorders. Med. Hypotheses. 64: 826–832 - PubMed
-
- de Ferranti S., Mozaffarian D. 2008. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 54: 945–955 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

