Genetic analysis of ORF5 of recent Korean porcine reproductive and respiratory syndrome viruses (PRRSVs) in viremic sera collected from MLV-vaccinating or non-vaccinating farms
- PMID: 19461207
- PMCID: PMC2801115
- DOI: 10.4142/jvs.2009.10.2.121
Genetic analysis of ORF5 of recent Korean porcine reproductive and respiratory syndrome viruses (PRRSVs) in viremic sera collected from MLV-vaccinating or non-vaccinating farms
Abstract
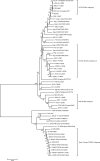
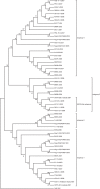
The 23 open reading frame (ORF) 5 sequences of Korean type II porcine reproductive and respiratory syndrome virus (PRRSV) were collected from viremic sera from the (modified live vaccine) MLV-vaccinating and non-vaccinating farms from 2007 to 2008. The samples were phylogenetically analyzed with previous ORF5 sequences, including type I Korean PRRSV, and previously reported or collected sequences from 1997 to 2008. A MN184-like subgroup of type II Korean PRRSV was newly identified in the viremic sera collected from 2007 to 2008. And of the type I PRRSVs, one subgroup had 87.2 approximately 88.9% similarity with the Lelystad virus, showing a close relationship with the 27 approximately 2003 strain of Spain. The maximum parsimony tree of type II PRRSV from 1997 to 2008 showed that they had evolved to four lineages, subgroups 1, 2, 3 and 4. Most of the recently collected type II PRRSVs belonged to subgroup 4 (48%). The region of three B-cell epitopes and two T-cell epitopes of ORF5 amino acids sequences was considerably different from the MLV in subgroups 3 and 4. In conclusion, the existence of type I PRRSV, which was genetically different from Lelystad virus (Prototype of type I PRRSV), and heterologous type II PRRSVs of viremic pigs detected even in the MLV-vaccinating farms indicated the need for new vaccine approaches for the control of PRRSV in Korea.
Figures

References
-
- Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000;145:2421–2429. - PubMed
-
- An DJ, Roh IS, Song DS, Park CK, Park BK. Phylogenetic characterization of porcine circovirus type 2 in PMWS and PDNS Korean pigs between 1999 and 2006. Virus Res. 2007;129:115–122. - PubMed
-
- Andreyev VG, Wesley RD, Mengeling WL, Vorwald AC, Lager KM. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch Virol. 1997;142:993–1001. - PubMed
-
- Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, Christianson WT, Morrison RB, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J Vet Diagn Invest. 1992;4:127–133. - PubMed
-
- Cano JP, Dee SA, Murtaugh MP, Pijoan C. Impact of a modified-live porcine reproductive and respiratory syndrome virus vaccine intervention on a population of pigs infected with a heterologous isolate. Vaccine. 2007;25:4382–4391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

