Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury
- PMID: 19461484
- PMCID: PMC2726263
- DOI: 10.1097/TP.0b013e3181a3c691
Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury
Abstract
Background: Hepatic ischemia reperfusion injury (IRI) is a major clinical problem during the perioperative period and occurs frequently after major hepatic resection or liver transplantation. Our laboratory previously demonstrated that exogenous A1 adenosine receptor activation protects against renal IRI by upregulation and phosphorylation of heat shock protein 27 (HSP27).
Methods: This study used mice overexpressing human HSP27 (huHSP27 OE) to determine whether these mice are protected against liver IRI.
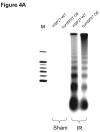
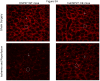
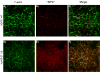
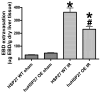
Results: After hepatic IR, the huHSP27 OE mice had significant protection against liver injury (reduced alanine transferase) and necrosis (hematoxylin-eosin staining) compared with the HSP27 WT mice. The huHSP27 OE mice also showed less induction of proinflammatory messenger RNA MIP-2, reduced neutrophil infiltration, and decreased apoptosis (caspase 3 fragmentation and DNA laddering) compared with the HSP27 WT mice. Finally, the huHSP27 OE mice showed significantly less disruption of filamentous actin in hepatocytes and bile canaliculi of the ischemic lobes compared with the HSP27 WT mice. Depletion of Kupffer cells with gadolinium chloride provided significant protection against liver IRI in HSP27 WT mice but not in huHSP27 OE mice suggesting that the overexpression of huHSP27 in the Kupffer cells may be responsible for the hepatic protection observed in huHSP27 OE mice.
Conclusions: Our results show that the overexpression of huHSP27 in Kupffer cells of the liver may be responsible for the protection against hepatic IRI in vivo by reducing necrosis and apoptosis and by stabilizing F-actin with subsequent reductions in inflammation and proinflammatory neutrophil infiltration. Harnessing the mechanisms of cytoprotection with HSP27 may lead to new therapies for the management of perioperative hepatic IRI.
Conflict of interest statement
Figures










Similar articles
-
Human heat shock protein 27-overexpressing mice are protected against acute kidney injury after hepatic ischemia and reperfusion.Am J Physiol Renal Physiol. 2009 Oct;297(4):F885-94. doi: 10.1152/ajprenal.00317.2009. Epub 2009 Aug 5. Am J Physiol Renal Physiol. 2009. PMID: 19656912 Free PMC article.
-
Selective renal overexpression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice.Am J Physiol Renal Physiol. 2010 Aug;299(2):F347-58. doi: 10.1152/ajprenal.00194.2010. Epub 2010 May 19. Am J Physiol Renal Physiol. 2010. PMID: 20484296 Free PMC article.
-
Locally synthesized HSP27 in hepatocytes: Is it possibly a novel strategy against human liver ischemia/reperfusion injury?Med Hypotheses. 2011 Feb;76(2):296-8. doi: 10.1016/j.mehy.2010.10.028. Epub 2010 Nov 9. Med Hypotheses. 2011. PMID: 21067867
-
A1AR-mediated renal protection against ischemia/reperfusion injury is dependent on HSP27 induction.Int Urol Nephrol. 2018 Jul;50(7):1355-1363. doi: 10.1007/s11255-018-1797-x. Epub 2018 Feb 26. Int Urol Nephrol. 2018. PMID: 29480441
-
Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion.Kidney Int. 2009 Mar;75(5):499-510. doi: 10.1038/ki.2008.572. Epub 2008 Nov 19. Kidney Int. 2009. PMID: 19020532 Free PMC article.
Cited by
-
Liver epithelial cells proliferate under hypoxia and protect the liver from ischemic injury via expression of HIF-1 alpha target genes.Surgery. 2012 Nov;152(5):869-78. doi: 10.1016/j.surg.2012.03.003. Epub 2012 May 8. Surgery. 2012. PMID: 22575885 Free PMC article.
-
Heat shock protein B1-deficient mice display impaired wound healing.PLoS One. 2013 Oct 15;8(10):e77383. doi: 10.1371/journal.pone.0077383. eCollection 2013. PLoS One. 2013. PMID: 24143227 Free PMC article.
-
Selective deletion of the endothelial sphingosine-1-phosphate 1 receptor exacerbates kidney ischemia-reperfusion injury.Kidney Int. 2014 Apr;85(4):807-23. doi: 10.1038/ki.2013.345. Epub 2013 Sep 11. Kidney Int. 2014. PMID: 24025642 Free PMC article.
-
Hepatocyte HSPA12A inhibits macrophage chemotaxis and activation to attenuate liver ischemia/reperfusion injury via suppressing glycolysis-mediated HMGB1 lactylation and secretion of hepatocytes.Theranostics. 2023 Jul 3;13(11):3856-3871. doi: 10.7150/thno.82607. eCollection 2023. Theranostics. 2023. PMID: 37441587 Free PMC article.
-
Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice.J Pharmacol Exp Ther. 2010 Jun;333(3):736-47. doi: 10.1124/jpet.110.166884. Epub 2010 Mar 22. J Pharmacol Exp Ther. 2010. PMID: 20308331 Free PMC article.
References
-
- Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. - PubMed
-
- Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. - PubMed
-
- Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. - PubMed
-
- Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 2006;69:233–238. - PubMed
-
- Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

