Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania
- PMID: 19461875
- PMCID: PMC2677456
- DOI: 10.1371/journal.ppat.1000441
Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania
Abstract
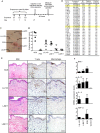
Immunity to a sand fly salivary protein protects against visceral leishmaniasis (VL) in hamsters. This protection was associated with the development of cellular immunity in the form of a delayed-type hypersensitivity response and the presence of IFN-gamma at the site of sand fly bites. To date, there are no data available regarding the cellular immune response to sand fly saliva in dogs, the main reservoirs of VL in Latin America, and its role in protection from this fatal disease. Two of 35 salivary proteins from the vector sand fly Lutzomyia longipalpis, identified using a novel approach termed reverse antigen screening, elicited strong cellular immunity in dogs. Immunization with either molecule induced high IgG(2) antibody levels and significant IFN-gamma production following in vitro stimulation of PBMC with salivary gland homogenate (SGH). Upon challenge with uninfected or infected flies, immunized dogs developed a cellular response at the bite site characterized by lymphocytic infiltration and IFN-gamma and IL-12 expression. Additionally, SGH-stimulated lymphocytes from immunized dogs efficiently killed Leishmania infantum chagasi within autologous macrophages. Certain sand fly salivary proteins are potent immunogens obligatorily co-deposited with Leishmania parasites during transmission. Their inclusion in an anti-Leishmania vaccine would exploit anti-saliva immunity following an infective sand fly bite and set the stage for a protective anti-Leishmania immune response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




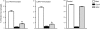
Similar articles
-
Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model.Proc Natl Acad Sci U S A. 2008 Jun 3;105(22):7845-50. doi: 10.1073/pnas.0712153105. Epub 2008 May 28. Proc Natl Acad Sci U S A. 2008. PMID: 18509051 Free PMC article.
-
Immunization of Experimental Dogs With Salivary Proteins From Lutzomyia longipalpis, Using DNA and Recombinant Canarypox Virus Induces Immune Responses Consistent With Protection Against Leishmania infantum.Front Immunol. 2018 Nov 16;9:2558. doi: 10.3389/fimmu.2018.02558. eCollection 2018. Front Immunol. 2018. PMID: 30519235 Free PMC article.
-
Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease.PLoS Negl Trop Dis. 2008 Apr 16;2(4):e226. doi: 10.1371/journal.pntd.0000226. PLoS Negl Trop Dis. 2008. PMID: 18414648 Free PMC article.
-
Sand fly saliva: effects on host immune response and Leishmania transmission.Folia Parasitol (Praha). 2006 Sep;53(3):161-71. Folia Parasitol (Praha). 2006. PMID: 17120496 Review.
-
Sand flies, Leishmania, and transcriptome-borne solutions.Parasitol Int. 2009 Mar;58(1):1-5. doi: 10.1016/j.parint.2008.07.004. Epub 2008 Aug 16. Parasitol Int. 2009. PMID: 18768167 Free PMC article. Review.
Cited by
-
Expression plasticity of Phlebotomus papatasi salivary gland genes in distinct ecotopes through the sand fly season.BMC Ecol. 2011 Oct 10;11:24. doi: 10.1186/1472-6785-11-24. BMC Ecol. 2011. PMID: 21985688 Free PMC article.
-
Canine antibody response to Phlebotomus perniciosus bites negatively correlates with the risk of Leishmania infantum transmission.PLoS Negl Trop Dis. 2011 Oct;5(10):e1344. doi: 10.1371/journal.pntd.0001344. Epub 2011 Oct 11. PLoS Negl Trop Dis. 2011. PMID: 22022626 Free PMC article.
-
Cytokine and Phenotypic Cell Profiles of Leishmania infantum Infection in the Dog.J Trop Med. 2012;2012:541571. doi: 10.1155/2012/541571. Epub 2011 Aug 9. J Trop Med. 2012. PMID: 21845197 Free PMC article.
-
Phlebotomus perniciosus Recombinant Salivary Proteins Polarize Murine Macrophages Toward the Anti-Inflammatory Phenotype.Front Cell Infect Microbiol. 2020 Aug 24;10:427. doi: 10.3389/fcimb.2020.00427. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32984064 Free PMC article.
-
RNA-sequencing of the Nyssomyia neivai sialome: a sand fly-vector from a Brazilian endemic area for tegumentary leishmaniasis and pemphigus foliaceus.Sci Rep. 2020 Oct 19;10(1):17664. doi: 10.1038/s41598-020-74343-y. Sci Rep. 2020. PMID: 33077743 Free PMC article.
References
-
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. - PubMed
-
- Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552–557. - PubMed
-
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. - PubMed
-
- Berman J. Visceral leishmaniasis in the New World & Africa. Indian J Med Res. 2006;123:289–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

