Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta
- PMID: 19461888
- PMCID: PMC2679197
- DOI: 10.1371/journal.ppat.1000444
Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta
Abstract
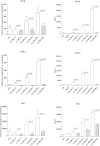
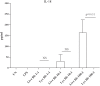
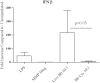
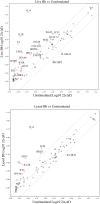
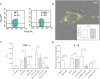
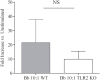
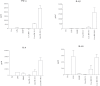
It is widely believed that innate immune responses to Borrelia burgdorferi (Bb) are primarily triggered by the spirochete's outer membrane lipoproteins signaling through cell surface TLR1/2. We recently challenged this notion by demonstrating that phagocytosis of live Bb by peripheral blood mononuclear cells (PBMCs) elicited greater production of proinflammatory cytokines than did equivalent bacterial lysates. Using whole genome microarrays, we show herein that, compared to lysates, live spirochetes elicited a more intense and much broader transcriptional response involving genes associated with diverse cellular processes; among these were IFN-beta and a number of interferon-stimulated genes (ISGs), which are not known to result from TLR2 signaling. Using isolated monocytes, we demonstrated that cell activation signals elicited by live Bb result from cell surface interactions and uptake and degradation of organisms within phagosomes. As with PBCMs, live Bb induced markedly greater transcription and secretion of TNF-alpha, IL-6, IL-10 and IL-1beta in monocytes than did lysates. Secreted IL-18, which, like IL-1beta, also requires cleavage by activated caspase-1, was generated only in response to live Bb. Pro-inflammatory cytokine production by TLR2-deficient murine macrophages was only moderately diminished in response to live Bb but was drastically impaired against lysates; TLR2 deficiency had no significant effect on uptake and degradation of spirochetes. As with PBMCs, live Bb was a much more potent inducer of IFN-beta and ISGs in isolated monocytes than were lysates or a synthetic TLR2 agonist. Collectively, our results indicate that the enhanced innate immune responses of monocytes following phagocytosis of live Bb have both TLR2-dependent and -independent components and that the latter induce transcription of type I IFNs and ISGs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes.J Leukoc Biol. 2013 Dec;94(6):1231-41. doi: 10.1189/jlb.0413206. Epub 2013 Aug 1. J Leukoc Biol. 2013. PMID: 23906644 Free PMC article. Clinical Trial.
-
Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta.Proc Natl Acad Sci U S A. 2011 Mar 1;108(9):3683-8. doi: 10.1073/pnas.1013776108. Epub 2011 Feb 14. Proc Natl Acad Sci U S A. 2011. PMID: 21321205 Free PMC article.
-
Live Borrelia burgdorferi spirochetes elicit inflammatory mediators from human monocytes via the Toll-like receptor signaling pathway.Infect Immun. 2009 Mar;77(3):1238-45. doi: 10.1128/IAI.01078-08. Epub 2009 Jan 12. Infect Immun. 2009. PMID: 19139200 Free PMC article.
-
Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi.J Immunol. 2002 Jan 1;168(1):348-55. doi: 10.4049/jimmunol.168.1.348. J Immunol. 2002. PMID: 11751980
-
Phagosomal TLR signaling upon Borrelia burgdorferi infection.Front Cell Infect Microbiol. 2014 May 20;4:55. doi: 10.3389/fcimb.2014.00055. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24904837 Free PMC article. Review.
Cited by
-
Inhibition of Borrelia Burgdorferi-Induced TLR2-NFκB Canonical Signaling by Gallic Acid through Targeting the CD14+ Adaptor Protein and p65 Molecule.Int J Mol Sci. 2022 Sep 20;23(19):10987. doi: 10.3390/ijms231910987. Int J Mol Sci. 2022. PMID: 36232290 Free PMC article.
-
Macrophage mediated recognition and clearance of Borrelia burgdorferi elicits MyD88-dependent and -independent phagosomal signals that contribute to phagocytosis and inflammation.BMC Immunol. 2021 May 17;22(1):32. doi: 10.1186/s12865-021-00418-8. BMC Immunol. 2021. PMID: 34000990 Free PMC article.
-
CD14 targets complement receptor 3 to lipid rafts during phagocytosis of Borrelia burgdorferi.Int J Biol Sci. 2013 Aug 20;9(8):803-10. doi: 10.7150/ijbs.7136. eCollection 2013. Int J Biol Sci. 2013. PMID: 23983613 Free PMC article.
-
Impact of E. muris infection on B. burgdorferi-induced joint pathology in mice.Front Immunol. 2024 Aug 20;15:1430419. doi: 10.3389/fimmu.2024.1430419. eCollection 2024. Front Immunol. 2024. PMID: 39229265 Free PMC article.
-
The infection-tolerant white-footed deermouse tempers interferon responses to endotoxin in comparison to the mouse and rat.Elife. 2024 Jan 9;12:RP90135. doi: 10.7554/eLife.90135. Elife. 2024. PMID: 38193896 Free PMC article.
References
-
- Salazar JC, Pope CD, Sellati TJ, Feder HM, Jr, Kiely TG, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–2670. - PubMed
-
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. - PubMed
-
- Feder HM, Jr, Johnson BJ, O'Connell S, Shapiro ED, Steere AC, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–1430. - PubMed
-
- Smith RP, Schoen RT, Rahn DW, Sikand VK, Nowakowski J, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136:421–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

