Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model
- PMID: 19461893
- PMCID: PMC2680948
- DOI: 10.1371/journal.pone.0005662
Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model
Abstract
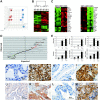
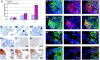
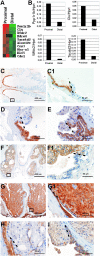
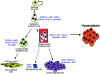
The PSA-Cre;Pten-loxP/loxP mouse prostate cancer model displays clearly defined stages of hyperplasia and cancer. Here, the initial stages of hyperplasia development are studied. Immunohistochemical staining showed that accumulated pAkt+ hyperplastic cells overexpress luminal epithelial cell marker CK8, and progenitor cell markers CK19 and Sca-1, but not basal epithelial cell markers. By expression profiling we identified novel hyperplastic cell markers, including Tacstd2 and Clu. Further we showed that at young age prostates of targeted Pten knockout mice contained in the luminal epithelial cell layer single pAkt+ cells, which overexpressed CK8, Sca-1, Tacstd2 and Clu; basal epithelial cells were always pAkt(-). Importantly, in the luminal epithelial cell layer of normal prostates we detected rare Clu+Tacstd2+Sca-1+ progenitor cells. These novel cells are candidate tumor initiating cells in Pten knockout mice. Remarkably, all luminal epithelial cells in the proximal region of normal prostates were Clu+Tacstd2+Sca-1+. However, in PSA-Cre;Pten-loxP/loxP mice, the proximal prostate does not contain hyperplastic foci. Small hyperplastic foci in prostates of PSA-Cre;Pten-loxP/+ mice found at old age, showed complete Pten inactivation and a progenitor marker profile. Finally, we present a novel model of prostate development and renewal, including lineage-specific luminal epithelial progenitor cells. It is proposed that Pten deficiency induces a shift in the balance of differentiation to proliferation in these cells.
Conflict of interest statement
Figures



References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. - PubMed
-
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

